ChemInform Abstract: Enantiocomplementary Routes to 6-Oxygenated (2Z,4E)-Alkadiene Precursors via Diastereoselective Reduction of 2-Cyclobuten-1-yl Ketones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
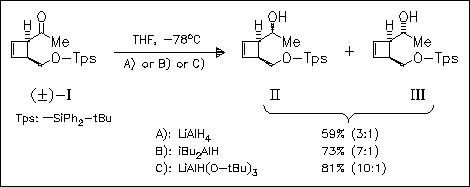
The ketone (I) and the hydroxy analogue are reduced with a series of hydride reagents in order to get alcohol products of type (II)/(III) with high diastereoselectivity. The best result is obtained for (I) in the presence of LiAlH(O-tBu)3. The products are precursors of (2Z, 4E)-alkadienals, suitable synthons for biologically active molecules.