ChemInform Abstract: Optically Active Dihydropyridines via Lipase-Catalyzed Enantioselective Hydrolysis.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
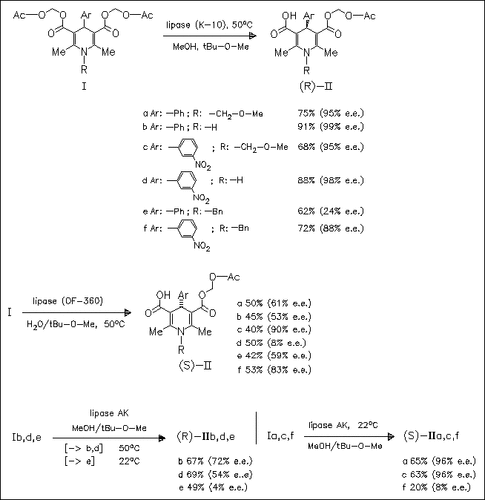
Hydrolysis of the prochiral diesters (I) in the presence of Pseudomonas lipases K-10 and P-30 leads to the (R)-monoesters (II), whereas with the Candida cylindracea lipase OF-360 the (S)-monoesters ( II) are obtained. In contrast, the stereochemical outcome of the Pseudomonas lipase AK mediated hydrolysis is found to depend on the substituents.