ChemInform Abstract: Asymmetric Sulfoxidation Using ((3,3-Dimethoxycamphoryl)sulfonyl) oxaziridine.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
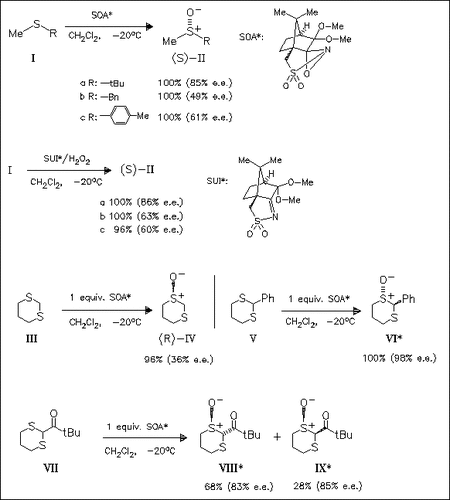
The title oxaziridine (SOA) is found to be an efficient agent for asymmetric oxidation of sulfides including dialkyl sulfides. Oxidation in the presence of ((3,3-dimethoxycamphoryl)sulfonyl)imine and H2O2 leads to similar results indicating that the oxaziridine (SOA) is the reactive intermediate.