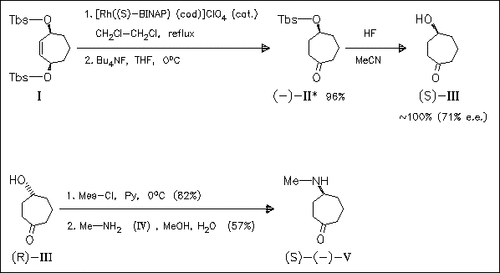
ChemInform Abstract: Catalytic Asymmetrization of meso-3,7-Bis(siloxy)cycloheptene by Chiral Rhodium(I)-binap (2,2′-Bis(diphenylphosphino)-1,1′-binaphthyl) Catalyst: The Enantiocontrolled Asymmetric Synthesis of (-)-(S)- Physoperuvine.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
Catalytic asymmetrization of the meso-bis(siloxy)cycloheptene (I) in the presence of a chiral rhodium (I)-BINAP catalyst provides the optically active siloxy ketone (cf. (II)) in ca. 70% e.e. after hydrolytic work-up. The (R)-enantiomer, obtained using the corresponding Rh-(R)-BINAP-catalyst, can be readily transformed into the alkaloid (S)-physoperuvine (V).