ChemInform Abstract: Enantiodivergent Synthesis of the Key Intermediate for Aphanorphine by Chemoenzymatic Process.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
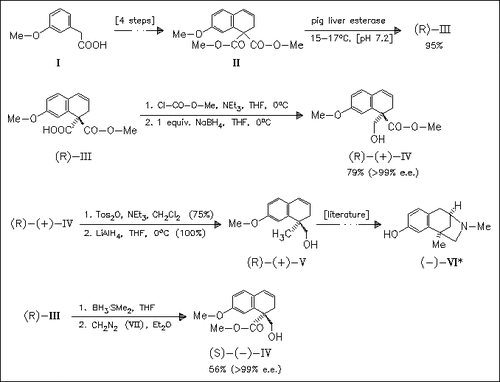
The synthesis of the key intermediate (R)-(V) of aphanorphine (VI) is achieved via enzymatic hydrolysis of the prochiral malonate (II) and selective reduction of the resulting acid ester (III). BH3×SMe2- mediated reduction of (III) followed by methylation gives the (S)- configurated alcohol (IV) which can be transformed into the enantiomeric intermediate (S)-(V).