ChemInform Abstract: Stereoselective Preparation of Alkyl Glycosides of 2-Acetamido-2-deoxy- α-D-glucopyranose by Nonclassical Halide-Ion Catalysis and Synthesis and NMR Spectroscopy of α-D-Gal p-(1 → 3)-. alpha.-D-Glc pNAc-OMe.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
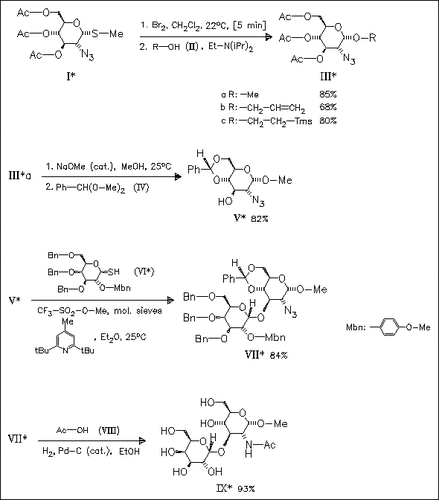
An efficient halide ion catalyzed stereocontrolled synthesis of simple glycosides such as (III) is reported. The methyl glycoside (IIIa) is converted into the title disaccharide (IX), which represents a part of the O-polysaccharide of the lipopolysaccharide of the human pathogen Shigella dysenteriae type 1.