ChemInform Abstract: Hydrolysis of the GlcNAc Oxazoline: Deamidation and Acyl Rearrangement.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
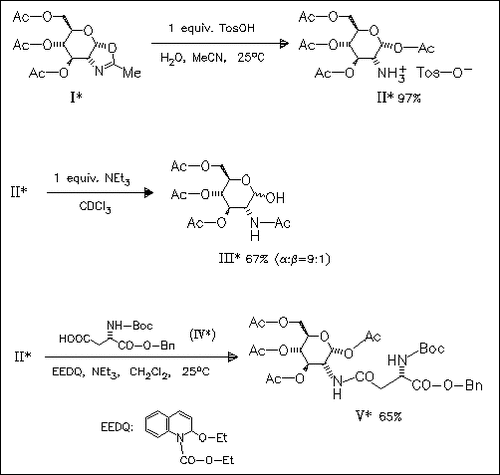
The specific deamidation of compound (I) under acidic conditions affords the p-toluenesulfonate (III), which undergoes base-catalyzed O- 1 → N-2 acyl rearrangement to give the 2-acetamido derivative ( III). Hydrolysis experiments of (I) show that the amino ester (II) represents the kinetic product, while amido alcohol (III) is the thermodynamic product. Acylation of (II) offers the route to novel glycoconjugates such as (V).