ChemInform Abstract: π-Facial Selectivity of the Singlet Oxygen (4 + 2) Cycloaddition to Chiral Naphthalene Derivatives: The Directing Effect of Carbon- Containing Substituents.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
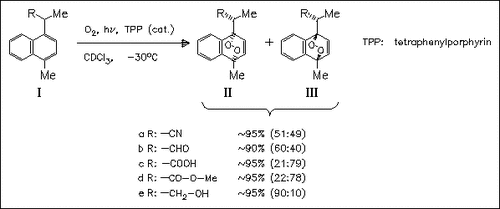
On photooxygenation the naphthalene derivatives (I) give the corresponding endoperoxides (II) and (III) in excellent yields. The diastereoselectivity of the singlet oxygen attack is determined and the directing effect of the various substituents R is rationalized in terms of attraction or repulsion in the transition state through electronic and steric effects.