ChemInform Abstract: Reagent Control of Cram-Type Selectivity in the Mukaiyama Aldol Catalyzed by Supersilylating Agents.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
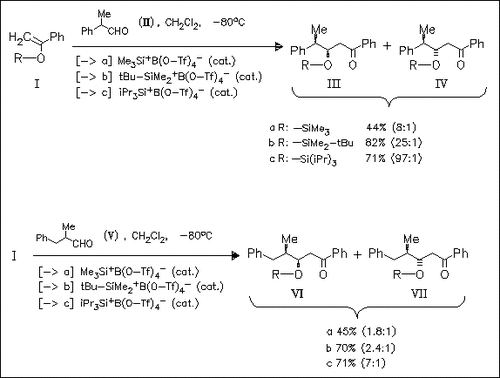
Supersilylating agents R3SiB(O-Tf)4 efficiently catalyze the Mukaiyama addition of enolsilanes (I) to unfunctionalized α-asym. aldehydes. The observed Cram-type selectivity correlates strongly with the steric bulk of the silyl group present in enolsilanes (I). Use of the triisopropylsilyl enol ether (Ic) results in unprecedented levels of 1,2-asym. induction.