ChemInform Abstract: The Use of Chiral Sulfides in Catalytic Asymmetric Epoxidation.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
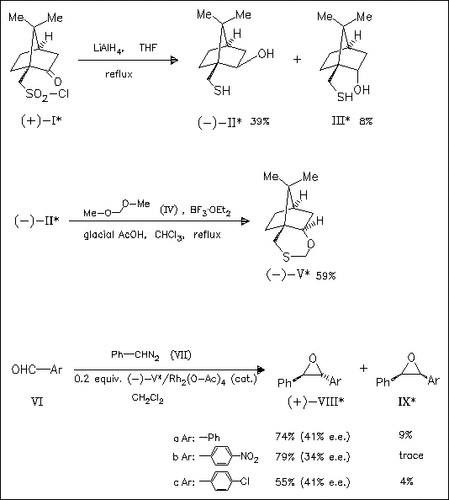
In the presence of the chiral sulfide (V), generated from (+)-10- camphorsulfonyl chloride, phenyl diazomethane reacts with aromatic as well as aliphatic aldehydes yielding oxiranes like (VIII) in good yield with reasonable enantioselectivity. Using its epimer resulting from the thiol (III) lower stereoselectivity is observed.