ChemInform Abstract: The Tetrahydropyranyl Group as a Chiral Auxiliary for the Nucleophilic Addition to α-Alkoxy Ketones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
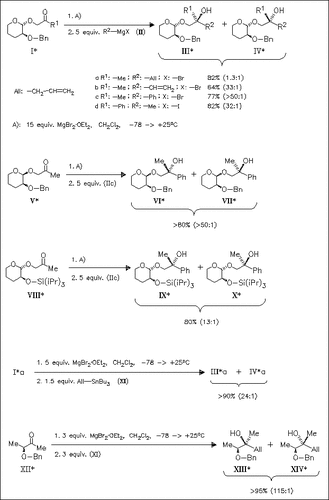
The protected 2-hydroxytetrahydropyranyl group is found to be an efficient chiral auxiliary for stereoselective nucleophilic addition of Grignard reagents to α-alkoxy ketones. Only in the case of the allyl derivative (IIa) a very low selectivity is observed. A significant increase, however, can be induced if the stannane (XI) is used instead of Grignard reagent (IIa). The synthetic utility of this method is further demonstrated in the reaction of ketone (XII).