ChemInform Abstract: Diastereoface Selectivity in Radical-Mediated C-C Bond Formation of Uridine 5′-Monoselenoacetals.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
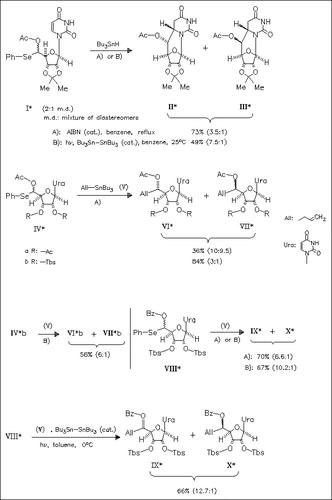
The intramolecular radical reaction of uridine-5′-monoselenoacetal (I) is shown not to follow Cram′s rule. Intermolecular versions of this reaction type are studied to demonstrate that both steric and electronic effects of the substituent of the oxygen determine a diastereoface selectivity (mechanism).