ChemInform Abstract: Enantiospecific Synthesis of ((2′S,3′S)-Bis(hydroxymethyl)azetidin-1- yl)pyrimidine Nucleosides as Potential Antiviral Agents.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
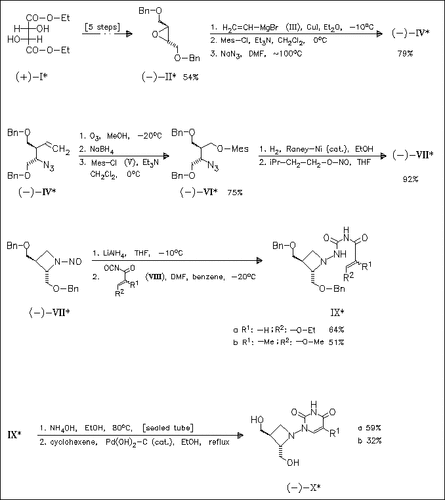
An efficient enatiospecific route to the title azetidinylpyrimidine nucleosides (cf. (X)), novel analogues of the potent antiviral antibiotic oxetanocin-A, is achieved. The key steps of the facile sequence are the construction of the azetidine base (VII) starting from optically active epoxybutane (II), coupling of the therefrom derived N-aminoazetidine with acryloyl isocyanates and subsequent ring closure.