ChemInform Abstract: Synthesis and Free Radical Scavenging Properties of the Enantiomers of Erdosteine.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
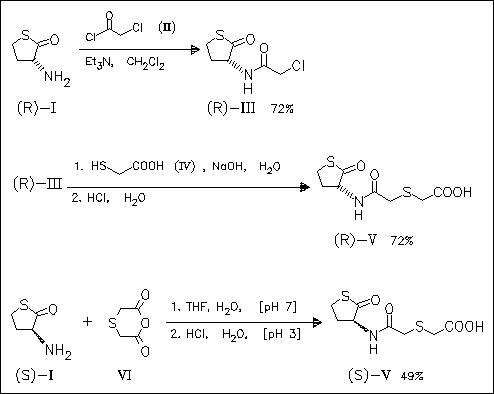
Both enantiomers of erdosteine (V) are synthesized on different routes. The (S)-isomer is shown to be more effective than the (R)-isomer in protecting mice against lethal doses of paraquat.