ChemInform Abstract: Synthesis of Furans by SN2′ Cyclization of γ-Alkynyl Allylic Alcohol Derivatives.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
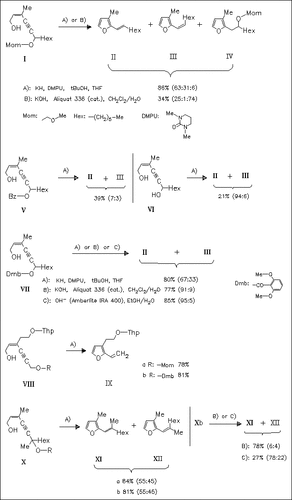
The reaction conditions of the recently described novel route to furans by base-catalyzed isomerization of γ-alkynyl allylic alcohols are optimized. The cosolvent 18-crown-6 is replaced successfully by the less toxic and less expensive additive DMPU in the reaction of the methoxymethyl ethers, e.g. (I) or (VIIIa). If milder conditions are required, the use of KOH/Aliquat 336 and 2,6- dimethoxybenzoates such as (VII) as starting compounds are the method of choice favoring strongly the formation of the E-isomers.