ChemInform Abstract: Evidence for Michael-Type Reaction of Alkenyliodonium Salts: Nucleophilic Substitutions with Sodium Benzenesulfinate.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
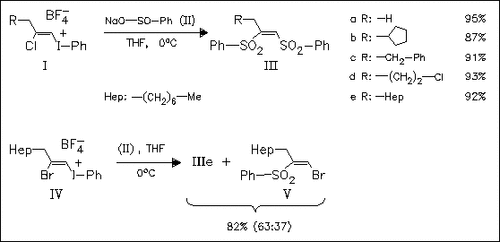
The nucleophilic vinylic substitutions of the tetrafluoroborates (I) with sodium benzenesulfinate (II) involve a hitherto unknown Michael- type addition to the Cβ-atom of (I). High yields of the bissulfones (III) are obtained. In contrast, application of the method to the bromo compound (IV) gives considerable amounts of the (Z)- halosulfone (V), a type of product which is obtained from (I) only in trace amounts.