ChemInform Abstract: Stereoselective Synthesis of Di- and Trisubstituted Alkenes via Intermolecular Addition of Vinyl Radicals to Alkenes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
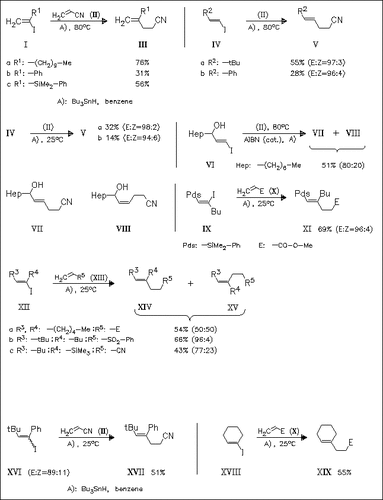
Different vinyl iodides are treated with Bu3SnH in the presence of several electron-poor alkenes in order to get insight into the scope of the reaction. The E/Z ratio of the products does not depend on the temperature; however, the yields at 25 °C are much lower than that found at 80 °C. On the other hand, the stereoselectivity is largely dependent on the substitution pattern of the starting iodides.