ChemInform Abstract: Double Asymmetric Induction in Intramolecular C-H Insertion Reactions of α-Diazo β-Keto Esters.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
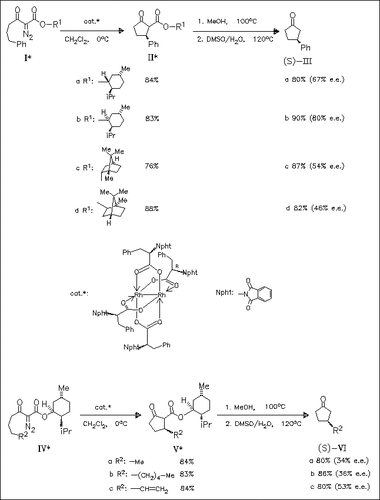
A new double asymmetric induction in C-C bond formation is achieved by using a homochiral rhodium catalyst in combination with a chiral ester group in the diazo keto esters (I) or (IV). Optically active 3- substituted cyclopentanones such as (III) or (VI) are obtained with up to 80% e.e. by using the neomenthyl esters (Ib) or (IV).