ChemInform Abstract: A Highly Selective Asymmetric Methoxyselenylation of Alkenes with a Chiral Ferrocenylselenium Reagent.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
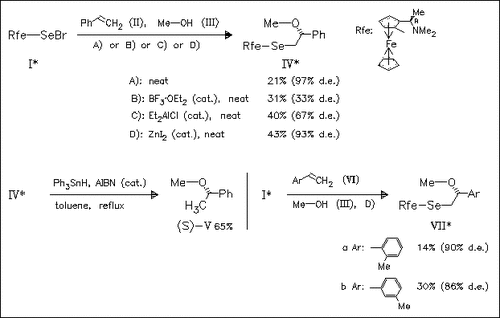
Freshly prepared chiral ferrocenylselenyl bromide (I) and its enantiomer react with alkenes in the presence of methanol according to D) with high facial selectivity to afford selenomethoxylated adducts.