ChemInform Abstract: Reactivity of 1-Aza-1,4,5,6-tetrahydroazulenes and Their Utility in the Preparation of Dipyrrolic Intermediates Needed for Petroporphyrin Synthesis.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
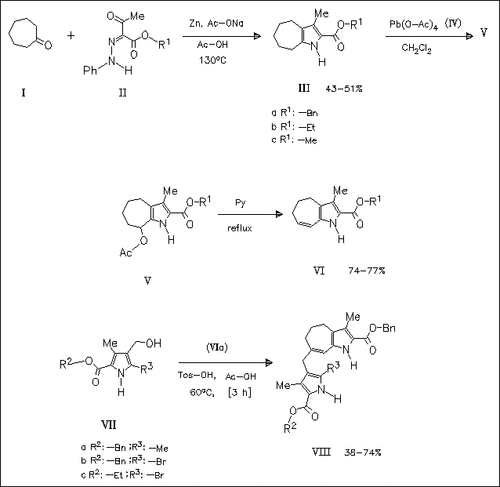
The azatetrahydroazulene (VIa) prepared from cycloheptanone (I) via the labile acetate (Va) undergoes coupling reaction with the pyrrole methanols (VII) under acidic conditions to yield the petroporphyrin synthons (VIII).