ChemInform Abstract: 5-Chloropyrazole-4-carbaldehydes as Synthons for Intramolecular 1,3- Dipolar Cycloadditions.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
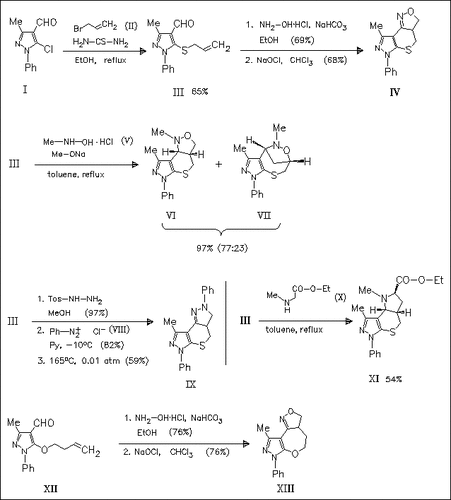
The title compound (I) is transformed into tricyclic heterocycles (IV), (VI), (IX) or (XI) in a short and attractive reaction sequence consisting of substitution of the chlorine atom, conversion of the aldehyde function into 1,3-dipoles and final intramolecular cycloaddition. Simultaneous Claisen rearrangement of the 5-allyloxy analogue of (III) limits its use for intramolecular 1,3-dipolar cycloaddition. Instead the homologous derivative (XII) can be used.