ChemInform Abstract: Enantioselective Synthesis of Allylic Alcohols via Asymmetric (2,3)- Sigmatropic Meisenheimer Rearrangement.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
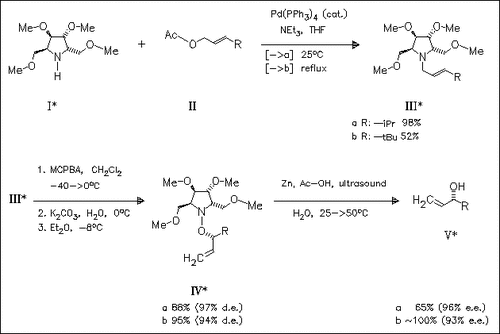
The N-oxides obtained from the chiral alkenylpyrrolidine (III) undergo Meisenheimer rearrengement to produce the hydroxylamine derivatives ( IV). Reduction of (IV) gives the allylic alcohols (V) of high enantiomeric purity.