ChemInform Abstract: (R)-1,2,2-Triphenyl-2-(trimethylsiloxy)ethyl Propionate: anti- Selective and Diastereofacially Selective Aldol Addition; Diastereoselective Silylation and Alkylation.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
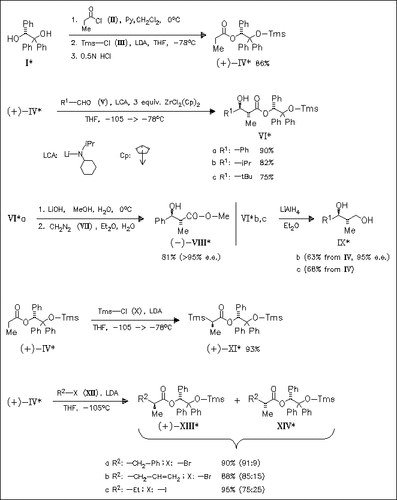
Aldol reactions of zirconium enolate derived in situ from readily obtained chiral title reagent (IV) proceed with high simple diastereoselection (anti:syn) as well as high diastereofacial selectivity to provide the anti-adducts (VI) in good yield. These adducts can be efficiently converted into enantiopure carboxylic acids (cf. (VIII)) and diols (cf. (IX)) thus demonstrating the synthetic potential of stereoselective aldol reaction induced by the chiral auxiliary triphenylglycol (I). Surprisingly, an exclusive diastereoselective carbon silylation occurs upon treatment of deprotonated ester (IV) with Tms-Cl. On the other hand, alkylation of the deprotonated ester (IV) with primary alkyl halides provides α -branched esters with moderate to acceptable diastereoselectivity (cf. (XIII)/(XIV)).