ChemInform Abstract: Highly Efficient Synthesis of 2-Substituted 4H-Chromen-4-ones by Means of F--Induced 6-endo-digonal Cyclization of o-(Silyloxy)phenyl Ethynyl Ketone Derivatives.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
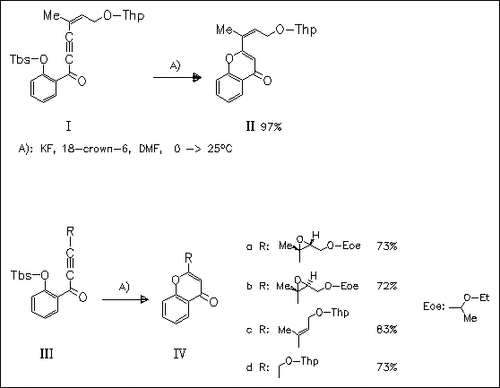
Under the optimized conditions A), cyclization of various ethynyl ketones is successful to give the title compounds in good to excellent yields. The method is simple and efficient and represents a general approach to 2-substituted 4H-chromen-4-ones, a segment present in the antitumor antibiotics kapurimycin A3 and altromycin.