ChemInform Abstract: Enantioselective Synthesis of Polyfunctional Small Building Blocks with a Quaternary Stereogenic Center.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
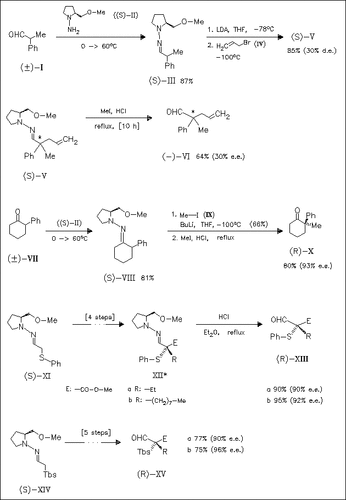
Application of the SAMP-/RAMP-hydrazone method offers an efficient and flexible access to compounds with a quaternary stereogenic center. As demonstrated in the scheme, rac. aldehydes such as (I) and ketones such as (VII) (11 examples) are easily transformed into the SAMP- hydrazones, which after alkylation and hydrazone cleavage yield the optically active aldehydes and ketones such as (VI) and (X). The method is extended to α-thiolated hydrazones (XI) (14 examples) and α-silylated hydrazones (XIV) (15 examples). They also produce quaternary stereogenic centers with high enantiomeric excess.