ChemInform Abstract: Ring Opening of Azetidinols by Phenols: Regiochemistry and Stereochemistry.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
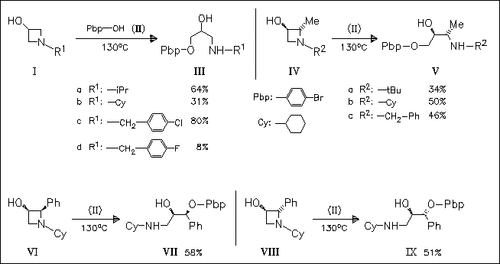
Ring opening of a series of 1-alkyl and 1-benzyl-3-azetidinols of type (I) by 4-bromophenol (II) without added base is reported. Opening of trans-2-methyl- (IV) and cis- and trans-2-phenyl-3-azetidinols (VI) and (VIII) is highly regioselective and occurs by cleavage of the N-C( 4) bond for (IV) and of the N-C(2) bond for (VI) and (VIII) involving inversion of configuration at C(2) with a high stereoselectivity. The results are rationalized in terms of nucleophilic ring opening of the azetidinium ions.