ChemInform Abstract: Substituent Control of Biscarbene Electronic Configurations: m- Phenylenebis(chloromethylene).
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
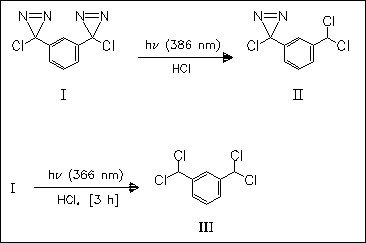
As part of a program aimed at an understanding of the factors that influence the electronic configurations in coupled reactive intermediates, the irradiation of the m-bisdiazirine (I) is examined. A new compound is formed which is trapped with HCl to give (II). Longer irradiation of (I) in a HCl-containing N2 matrix leads to (III), which is also obtained from (II). These studies reveal that both mono- and bis-carbenes are generated. The title carbene is a closed-shell ground-state singlet, which is robust to maintain its identity when meta-coupled.