ChemInform Abstract: Evidence for Selective S-Alkylation of an Ambident Anion of Dicyclohexylammonium Thiophosphonate by Alkyl Halides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
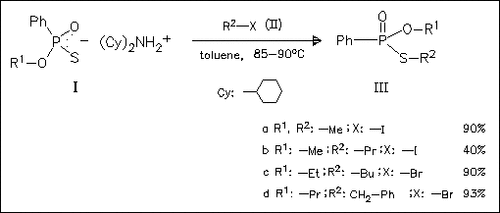
The title reaction between (I) and (II) gives exclusively S- derivatives of type (III). The cause for poor yields of the O-methyl analogues (R2: -Pr, -Bu, -CH2-PH) (cf. (IIIb)) is their ability to participate in the methylation of the salt (Ia) giving rise to (IIIa) and dicyclohexylammonium salts of S-alkyl phenylphosphonothioic acids as by-products. The proposed mechanism for the formation of (III) involving a transition state of the SN2 type is confirmed by synthesis of pure enantiomers of the model compound (IIIc).