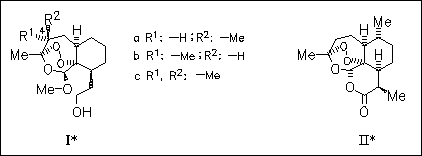ChemInform Abstract: Mechanism-Based Design, Synthesis, and in vitro Antimalarial Testing of New 4-Methylated Trioxanes Structurally Related to Artemisinin: The Importance of a Carbon-Centered Radical for Antimalarial Activity.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
Three trioxanes (I) are synthesized to evaluate their antimalarial potencies in vitro against both chloroquine-resistant and chloroquine- susceptible strains of Plasmodium falciparum. Mechanistic studies illustrate the influence of stereochemistry and support the suggestion, that antimalarial activity depends on the possibility of the molecule to undergo 1,5-hydrogen atom transfer specifically of 4α-H to generate a carbon-centered radical, which is then metabolized. Thus, only 4β-methylated trioxane (Ia), which can undergo 1,5-hydrogen atom transfer, shows high in vitro antimalarial activity and is even more potent than artemisinin (II).