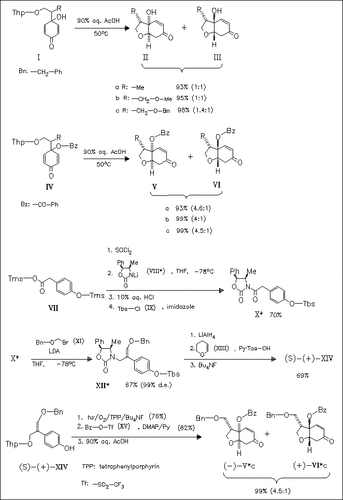
ChemInform Abstract: Diastereotopic Group Selective Intramolecular Conjugate Addition of 4-( 2-Hydroxyethyl)-p-quinol Derivatives: Synthesis of the Optically Pure cis-7-Oxabicyclo(4.3.0)non-2-en-4-one Skeleton.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The asymmetric syntheses of the optically pure oxabicyclononenone (-)-( Vc), which is a useful intermediate in the synthesis of optically active avermectins, and its enantiomer are carried out by cyclization of the p-quinol derivatives, first studied for the racemic compounds, such as (I) or (IV).