ChemInform Abstract: Macrolactonization-Transannular Aldol Condensation Approach to the Taxane AB Ring System.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
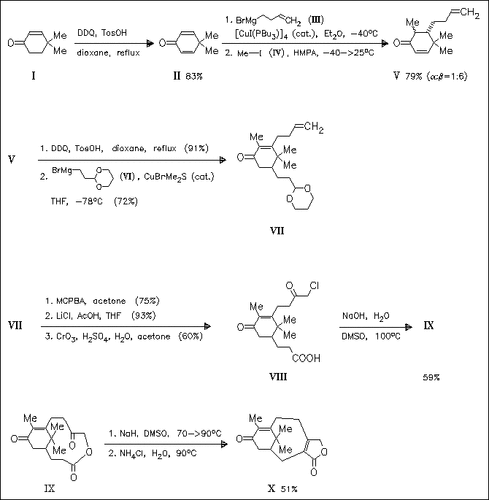
The 2(5H)-furanone (X) which is an important intermediate in the synthesis of the antimitotic taxane diterpenoid taxol is synthesized in a nine step-synthesis from cyclohexenone (I). Key steps of the reaction are two copper-catalyzed Grignard addition reactions for producing the taxane A ring in compound (VII). The eight-membered taxane B ring is introduced by a macrolactonization-transannular aldol condensation protocol of (VII).