ChemInform Abstract: A Novel Procedure for the Synthesis of α,β-Disubstituted . beta.-Fluorovinyl and β-Trifluoromethylvinyl Sulfides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
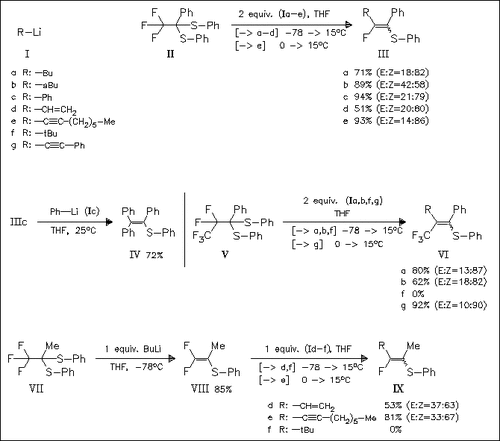
Treatment of the phenylsubstituted sulfides (II) and (V) with organolithium compounds (I) affords directly the vinyl sulfides (III) and (VI). However, 2 equiv. of (I) are required for complete reaction. Product (IIIc), on further treatment with (Ic), yields the trisubstituted sulfide (IV), an important synthon in the preparation of mammary tumor inhibitors. The methylsubstituted sulfide (VII), when treated with BuLi, yields the difluorovinyl sulfide (VIII), a type of compound which is assumed to be the intermediate species in the reactions (II) → (III) and (V) → (VI). Reaction of (VIII) with (I) finally gives the vinyl sulfides. The above methodology thus represents a general approach to the title compounds.