ChemInform Abstract: Regioselective Introduction of Functional Groups in α-Diimines by Means of Dialkylzinc Compounds. Synthesis of Functionalized 2- and 3-Pyrrolidinone Derivatives.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
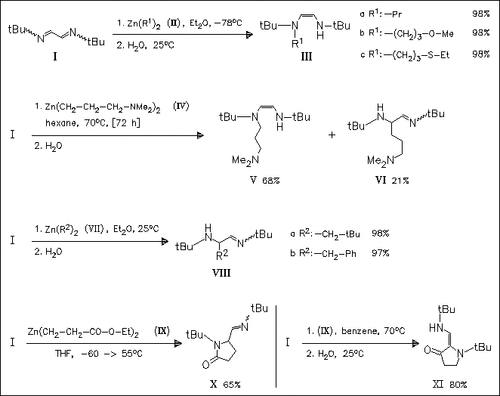
Alkylation of the diazabutadiene (I) by functionally substituted diorganozinc compounds occurs regioselectively. Only N-alkylations is observed when the Zn compounds have prim. substituents such as in (II), while C-alkylation takes place when sec. or benzylic groups are attached to Zn as in (VII). The functional groups in the Zn reagent proves to important only in the case of (IV), where the strongly coordinating NMe2 group causes a decrease of the alkylation rate leading to a mixture of (VI) and (VII) together with 11% of unknown compounds, and in the case of Zn(o-C6H4-CH2-NMe2)2, where no reaction occurs. In the reaction with (IX), depending on the conditions used, different pyrrolidinones are obtained in a two-step process involving the regioselective N- or C-alkylation followed by an intramolecular nucleophilic substitution.