ChemInform Abstract: Reaction of Carbenes with Cyclic Ethers in the Presence of Nucleophiles. A Three-Component Coupling Reaction.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
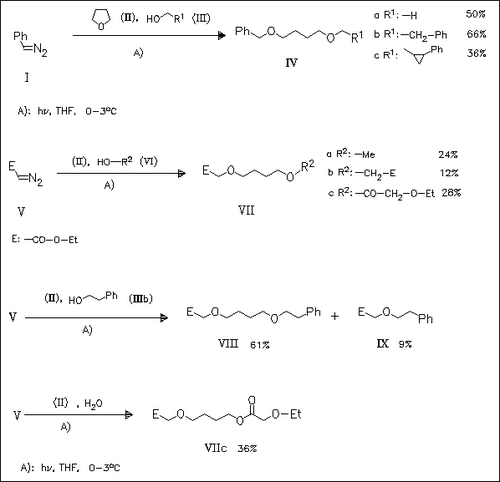
The coupling reaction of carbenes, generated photolytically from the corresponding diazo derivatives such as (I) and (V), with THF (II) or tetrahydropyran and different nucleophiles such as alcohols, thiols, carboxylic acids, amines, and H2O proceeds via cyclic oxonium ylides. The reaction mechanism including the by-product formation is described; thus, the formation of (VIIc) with water is rationalized by an attack of the rearranged OC=CH-OEt species.