ChemInform Abstract: Syntheses of Racemic and Both Chiral Forms of Cyclopropane-1,2-d2 and Cyclopropane-1-13C-1,2,3-d3.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
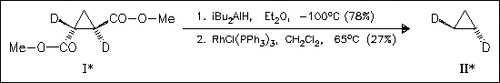
The racemic and both chiral forms of title compounds such as (II) are prepared through sequences based on trans-1,2-bis-(methoxycarbonyl) cyclopropanes such as (I). The synthesis of these diesters in racemic form (with the corresponding labeling patterns) is described. Optical resolution is achieved via selective hydrolysis of one ester group by pig liver esterase followed by chromatographic separation of the diastereomeric amides prepared from (R)-2-phenylglycinol.