ChemInform Abstract: An Effective Combination of Triphenylphosphine and Benzyl Azide for O- and N-Alkylations of Carboxylic Acids and Imides with Alcohols.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
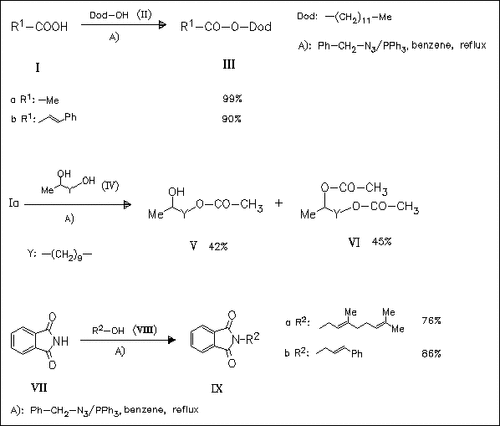
The esterification, as is demonstrated for (III), is examplified with 4 alcohols and 4 acids. The steric bulkiness is an important factor in the reaction; however, primary alcohols are more preferable, whereas tertiary alcohols do not react. The difference of reactivity is also shown with the alcohol (IV). Imides such as (VII) with an acidic proton are N-alkylated by this method as is demonstrated for (IX) (8 examples). However, only primary alcohols give good yields.