ChemInform Abstract: Addition Reactions of 5-Aminobenzotriazoles with Dimethyl Acetylenedicarboxylate (DMAD). Formation of Z/E-Michael Adducts, ( Benzotriazol-5-yl)-2-pyridones, a Triazolo-9,10-dihydrobenzo(b)azepine and a Triazolo-2-oxindole.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
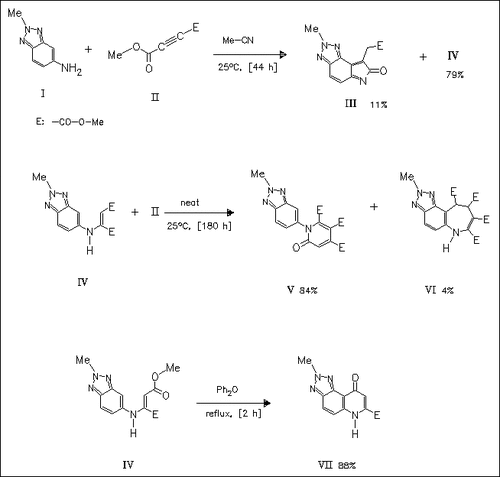
5-Aminobenzotriazoles such as (I) are coupled with dimethyl acetylenedicarboxylate (II) to yield the Michael adduct (IV) as the main product. This is further converted to the 2-pyridone (V), accompanied by the azepine derivative (VI), or to the quinolinone (VII) .