ChemInform Abstract: Enzymatic Enantioselective Ester Hydrolysis by Carboxylesterase NP.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
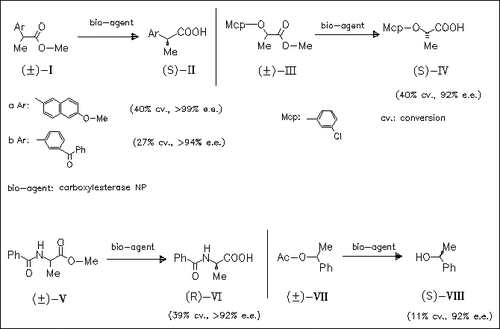
The enzymatic hydrolysis of a series of racemic carboxylic methyl and ethyl esters by carboxylesterase NP is investigated in order to determine the scope and limitations of this enzyme. Hydrolysis with high enantioselectivity is achieved with several 2-arylpropionates, 2-( aryloxy)propionates, and N-arylalanine esters such as (I), (III), and ( VI) producing propionic acids as (S)-enantiomers and alanine derivatives as (R)-enantiomers. The enzymatic hydrolysis of propionates without 2-aryl substituents occurs at a lower rate without acceptable enantioselectivity. 1-Phenylethyl acetate (VII) is also hydrolyzed enantioselectively. Carboxylesterase NP proved to be a powerful enzyme for kinetic resolution of 2-substituted propionate esters containing an aromatic moiety.