ChemInform Abstract: Asymmetric Synthesis of Sulfinimines: Applications to the Synthesis of Nonracemic β-Amino Acids and α-Hydroxy β-Amino Acids.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
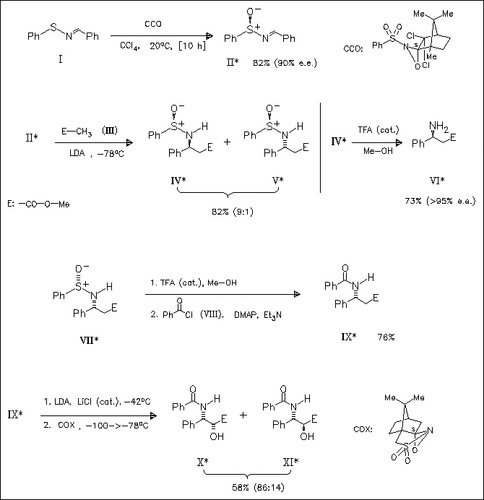
Asymmetric oxidation of the sulfenimine (I) using a chiral oxaziridine produces the sulfinimine (II). This optically active ammonia synthon is coupled with the lithium enolate of methyl acetate (III). Subsequent chromatographic purification and acidic deprotection of (IV) gives the β-amino acid ester (VI). Furthermore, the enantiomer ( VII) of (IV) is converted to the N-benzoyl derivative (IX) which is subjected to oxidation with (+)-(camphorsulfonyl)oxaziridine to yield the α-hydroxy β-amino acid derivatives (X) and (XI).