ChemInform Abstract: Asymmetric Twin-Ring Differentiation by Lipase-Catalyzed Enantiotoposelective Reaction of the Ring-Crossed meso Glycol: Asymmetric Synthesis of a Highly Functionalized Piperidine from the Conjoined Twin Piperidine System.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
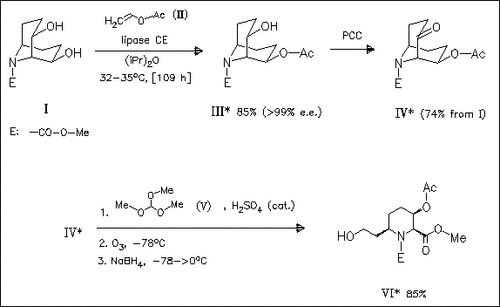
Lipase-catalyzed transesterification reaction of the diol (I) with vinyl acetate (II) affords the almost optically pure chiral monoacetate (III) which is further oxidized to give the ketone (IV). This ketone (IV) is cleaved to form the derivative (VI) of the desired chiral piperidine mentioned in the title. The enantiomer of (VI) is obtained via lipase catalyzed selective hydrolysis of the diacetate.