ChemInform Abstract: Generation and Characterization of 1,3-Di-tert-butyl- and 1,3-Di-tert- butyl-4,6-dimethylthieno(3,4-c)thiophenes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
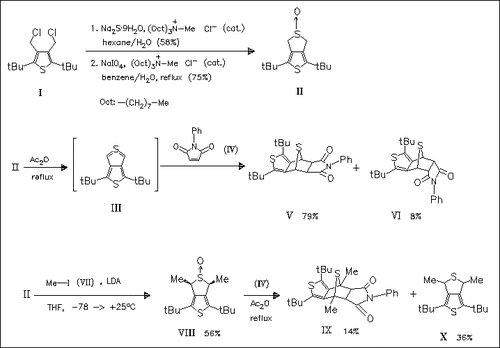
The unstable title compound (III) and the 4,6-dimethyl derivative are generated by Pummerer dehydration of the corresponding sulfoxides (II) and (VIII) in refluxing acetic anhydride and are trapped with N- phenylmaleimide (IV). Both exo- and endo-adducts (V) and (VI) are formed from (III), while only the exo-adduct (IX) is formed from the 4, 6-dimethyl analogue in addition to the reduction product (X). Dehydration of (II) and (VIII) with trifluoroacetic anhydride and lutidine lead to reduction and isomerization. In order to isolate a sterically stabilized thieno(3,4-c)thiophene, introduction of more bulky alkyl groups is necessary.