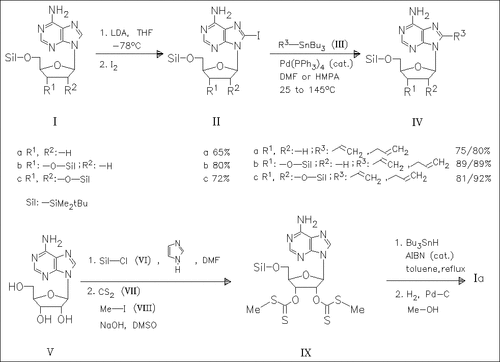
ChemInform Abstract: Palladium-Catalyzed C-8 Allylation and Vinylation of Adenosine, 2′- Deoxyadenosine, and 2′,3′-Dideoxyadenosine Nucleosides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The (deoxy)adenosines (I) are consecutively treated with LDA and iodine to form the 8-iodo derivatives (II) which are subjected to substitution reaction with vinyl- or allyltributylstannane (III) in the presence of a Pd(0) catalyst to give the 8-alkenyl derivatives (IV) . The synthesis of (Ia) from the adenosine (V) is described.