ChemInform Abstract: Synthesis and Complexation Properties of a Water-Soluble Optically Active Cyclophane Incorporating a 4-Naphthyl-1,2,3,4- tetrahydroisoquinoline Unit as a Chiral Spacer.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
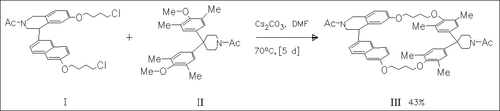
Cyclization of the chiral naphthalenyltetrahydroisoquinoline derivative (I) with the bis(hydroxyphenyl)piperidine derivative (II) yields the optically active macrocycle (III). After reduction of the two N-acetyl groups in (III) with BH3×THF and subsequent quaternization of the two tertiary amino groups with ethyl iodide and ion exchange of iodide to chloride, a macrocyclic dication is obtained which shows modest chiral recognition in the inclusion complexation of naproxen derivatives.