ChemInform Abstract: Regiospecific Acylation Reactions of Imidazo(1,5-a)pyridine.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
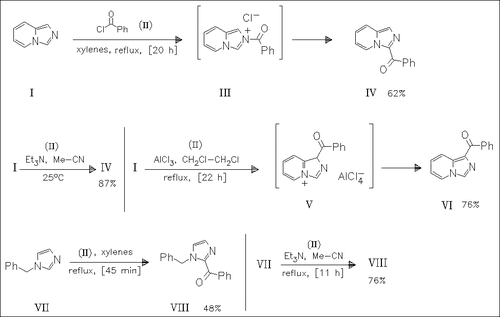
The imidazopyridine (I) is subjected to reaction with benzoyl chloride (II) under various conditions. Heating in xylenes or treatment with triethylamine/acetonitrile result in the formation of the salt (III) which is rearranged to give the 3-benzoyl derivative (IV). Friedel—Crafts acylation of (I) with (II), however, produces the 1-benzoylimidazopyridine (VI) via the intermediate salt (V). An isomerization process between (IV) and (VI) is not observed. The N-benzylimidazole (VII) is transformed into the 2-benzoylimidazole (VIII).