ChemInform Abstract: Synthesis of a New Family of Chiral Fluorinated Synthons: (R)- and (S)-4-Fluoro-1-alkynes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
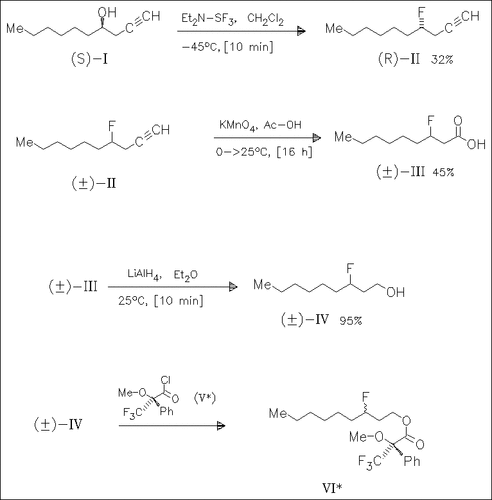
Reaction of optically pure 1-decyn-4-ol (R)-(I) with diethylaminosulfur trifluoride gives the 4-fluoro-1-decyne (R)-(II). The corresponding racemate (.+-.)-(II) is oxidized to yield the acid (.+-.)-(III). Subsequent reduction produces the alcohol (.+-.)-(IV) which is coupled with the chiral acid chloride (V) to form a mixture of the diastereomers (VI). This might be separated and cleaved to give the chiral alcohols.