ChemInform Abstract: Synthesis of Enantiomerically Pure Amino Acids Containing a 2-Amino-4- thiazolyl Side Chain.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
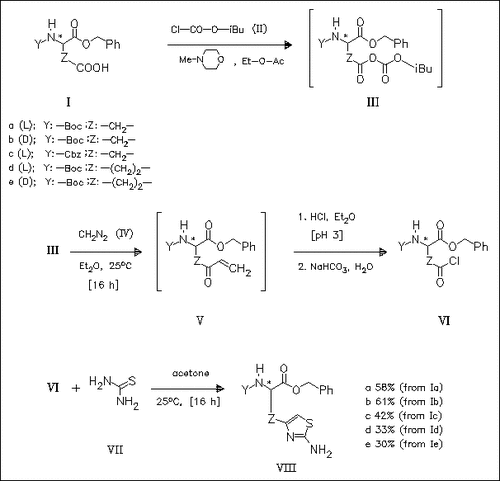
The aspartic or glutamic acid derivatives (I) are converted to the diazo ketones (V) via the mixed anhydrides (III). Degradation of (V) by treatment with hydrochloric acid gives the crude α-chloro ketones (VI) which undergo ring closure reaction with thiourea (VII), forming the protected thiazolyl-substituted amino acids (VIII).