ChemInform Abstract: Broadening in the Scope of NADH Models by Using Chiral and Non-Chiral Pyrrolo(2,3-b)pyridine Derivatives.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
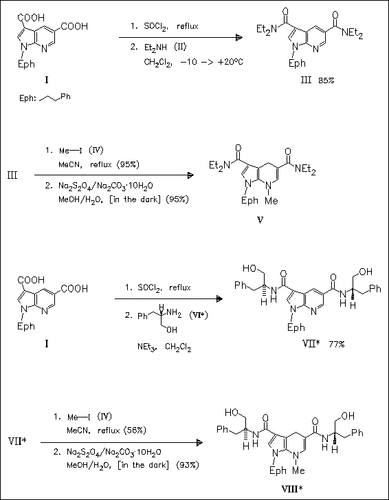
In comparison to the hitherto known NADH coenzyme models the novel title compounds (V) and (VIII) allow the reduction of substrates such as benzaldehydes, araliphatic ketones, 1-exo-methyleneisoquinoline or cyclohexanone in generally high yields. Furthermore the stereochemistry of the reduction product of a prochiral ketone is not fixed and, depending on the reaction conditions either of the desired enantiomers can be prepared. Finally, the title compounds can be used in the syntheses of chiral precursors of target molecules, which are produced in high enantiomeric excesses.