ChemInform Abstract: α-Sulfinyl-Substituted Radicals. Part 1. Stereoselective Radical Addition Reactions of Cyclic α-Sulfinyl Radicals.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
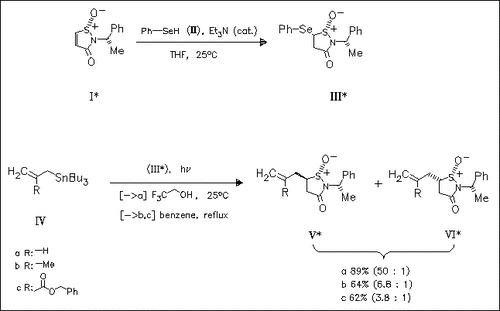
Enantiomerically pure 3-oxo-2-(1-phenylethyl)-5-isothiazolidinyl 1-oxide radicals, prepared from the 5-phenylseleno compound (III), undergo addition reactions with the (2-alkenyl)tributylstannanes (IV) to give the 5-(2-alkenyl)-2-(1-phenylethyl)-3-isothiazolidone 1-oxides (V) and (VI) with predominance of the former. The diastereoselectivity of these radical additions proves to depend on the hydrogen bridging ability of the solvent. Further examples are described in the original paper.