Research progress on electronic and active site engineering of cobalt-based electrocatalysts for oxygen evolution reaction
Chuansheng He and Linlin Yang contributed equally to this study.
Abstract
Electrocatalytic water splitting has been identified as a potential candidate for producing clean hydrogen energy with zero carbon emission. However, the sluggish kinetics of oxygen evolution reaction on the anode side of the water-splitting device significantly hinders its practical applications. Generally, the efficiency of oxygen evolution processes depends greatly on the availability of cost-effective catalysts with high activity and selectivity. In recent years, extensive theoretical and experimental studies have demonstrated that cobalt (Co)-based nanomaterials, especially low-dimensional Co-based nanomaterials with a huge specific surface area and abundant unsaturated active sites, have emerged as versatile electrocatalysts for oxygen evolution reactions, and thus, great progress has been made in the rational design and synthesis of Co-based nanomaterials for electrocatalytic oxygen evolution reactions. Considering the remarkable progress in this area, in this timely review, we highlight the most recent developments in Co-based nanomaterials relating to their dimensional control, defect regulation (conductivity), electronic structure regulation, and so forth. Furthermore, a brief conclusion about recent progress achieved in oxygen evolution on Co-based nanomaterials, as well as an outlook on future research challenges, is given.
1 INTRODUCTION
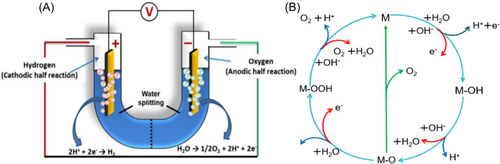
Clean and renewable energy development plays a pivotal role in replacing fossil fuels and addressing energy crisis and pollution issues.1, 2 Notably, researchers have made significant progress in devising energy storage and conversion devices, including metal-air batteries, fuel cells, lithium-ion batteries, and water-splitting reactors.3-8 Among these devices, electrochemical water splitting to generate hydrogen energy stands out as a clean and efficient method, garnering widespread attention in various catalytic conversion technologies, especially amidst the ongoing social energy crisis.9 The electrochemical water splitting reaction can be generally categorized into two main reactions, as shown in Figure 1A: the oxygen evolution reaction (OER) occurs on the anode, and the hydrogen evolution reaction (HER) takes place on the cathode.12-15 However, a fundamental difference exists between these two reactions: the OER involves a complex four-electron transfer pathway, while the HER involves only a simple two-electron process. As a consequence of this disparity, OER is greatly hindered by slower kinetics and requires a larger overpotential compared to the HER, making it the rate-limiting step in the water-splitting process.10, 16
Extensive studies have shown that properly designed catalysts are crucial to improving OER performance. The design principles for OER catalysts usually encompass several key aspects. (i) Optimal elemental composition. Commercially, ruthenium oxide (RuO2) or iridium oxide (IrO2) catalysts remain the conventional options for electrocatalytic OER to speed up its kinetics, while their high cost or poor stability seriously limits their commercial development process.6, 17-20 Therefore, transition metals such as cobalt (Co) with low cost (the price of Ru metal and Ir metal is about 500 times and 5000 times that of transition Co metal),21, 22 which have available d orbitals and active d electrons, are more efficient to adsorb oxygen intermediates and hybrid with p orbitals of O. Therefore, Co-based nanomaterial (NM) could be the alternative candidate to achieve highly efficient OER catalytic performance. Besides, to achieve practical applications, catalysts are required to use abundant and cost-effective materials to enable scalable and economically viable OER processes. (ii) Maximization of active sites. Manipulating the catalysts' structure into low dimensions ranging from zero-dimensional nanoparticles to one-dimensional nanowires, two-dimensional nanosheets (NSs), and three-dimensional self-supported electrodes significantly increases the number of active sites available on the catalysts' surface, thereby enhancing their catalytic activity. (iii) Electronic structure engineering. Tailoring the electronic structure of the catalysts is an efficient strategy to facilitate the OER performance. This can be achieved through the adjustment of the crystal structures, manipulation of coordination environments, introduction of heteroatoms, or construction of composite catalysts with conductive materials. Additionally, incorporating vacancy effects, such as anion or cation vacancies, optimizes the adsorption/desorption ability of OER intermediates and enhances the conductivity to achieve efficient charge transfer pathways, which can decrease energy losses.
The special electron configuration of the first-row transition metal enables an effective hybridization between electrons from the d orbitals of the metal and the p orbitals of the O from the water molecule or an oxygen-containing intermediate, leading to efficient d electron transfer and, thus, achieving outstanding OER performance.23 Among them, Co-based catalysts have garnered extensive research attention due to their abundant reservation, relatively inexpensive price, and potential economic feasibility for large-scale applications,23, 24 and they have been proven to be highly efficient OER catalysts.19, 25-29 The valence electron layer of Co is 3d74s2, and after losing the outer electron, it has abundant d electronic and empty orbitals, which can participate in the adsorption and desorption reactions of substances, providing a potential possibility for catalytic electrochemical reaction.
Recently, Co-based catalysts, such as Co oxides,30-32 hydroxides,33-35 selenides,36, 37 phosphates,38-40 and sulfides,41-43 have been widely studied as OER catalysts. CoO is generally composed of a cubic phase with Co2+ filled in the crystal cell structure, and meanwhile, Co2+ ion is proved to be more catalytically active than Co3+ ions in spinel-structured Co oxides.44 Besides, Co3O4 has shown adjustable electrocatalytic properties due to its spinel structure, in which Co2+ and oxygen atoms are combined to form a CoO4 tetrahedral structure, and Co3+ and oxygen atoms are combined to form a CoO6 octahedral structure.45, 46 Furthermore, Co(OH)2-layered double hydroxides consist of alternating layers composed of Co ions (Co2+) and hydroxide ions (OH−),47 and this coordination geometry structure is favorable for related ion exchange capabilities and adsorption behavior for OER. In addition, Co-based selenides,48-51 such as CoSe with layered tetragonal or nonlayered hexagonal structure and CoSe2 with an orthorhombic structure, and so forth,50, 51 have good electrical conductivity because they have a smaller band gap that facilitates efficient charge transport pathways for OER. Moreover, Co phosphides, such as Co2P and CoP, also attracted more attention for OER, which is attributed to their cubic, hexagonal crystal structures.52-57 During the OER process, Co-based catalysts may undergo surface reconstruction. For Co oxides and hydroxides, the surface may undergo a sequential decomposition process from Co-O, Co-OH to CoOOH on the catalyst's surface.58 For Co selenides (CoSex/Co-O, CoSex/Co-OH, and CoSex/Co-OOH) or Co phosphides (CoxP/Co-O, CoxP/Co-OH, and CoxP/Co-OOH), active intermediates species may form successively and be involved in the OER reaction. This reconstructed heterojunction plays a crucial role in adjusting the electronic states of the catalysts to promote the OER performance.58
Various strategies, such as defect introduction, dimensional regulation, crystal phase regulation, heterojunction construction, and combination of catalysts with conductive support, have been proposed to enhance the catalytic performance (Figure 2).45, 58-62 Among them, low-dimension materials have attracted much attention due to their large surface area, improved mass transport, and tunable electronic structure.11, 63, 64 Introducing defects or vacancies into Co-based catalysts can create more active sites and modify the electronic structure, leading to improved OER catalytic activity.65-67 Exposing different crystal directions/facets or designing amorphous structures in electrocatalysts can boost OER performance due to the unique exposed active sites and altered surface properties,68-70 originating from the different surface atomic arrangements, electronic structure, and surface energies.71 Co-based catalysts with designed heterostructure show efficient charge separation and transportation, thus enhancing OER kinetics.72 Incorporating Co-based catalysts onto suitable supports, such as carbon materials, metal oxides, or conductive polymers, can efficiently prevent active site aggregation and facilitate electron transfer, resulting in improved OER performance.73-75
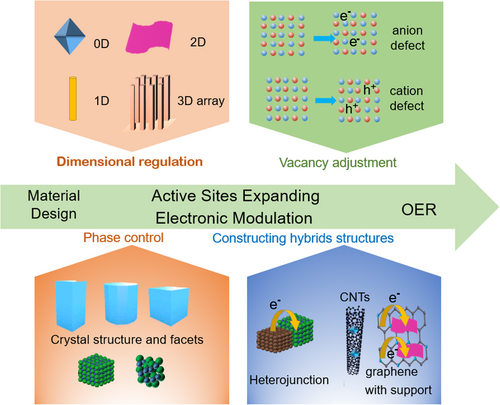
Recent intensive studies for electrochemical OER have led to the development of various promising Co-based electrocatalysts. Although several review articles regarding synthesis methods and simple descriptions of various Co-based catalysts for OER have been published, as far as we know, none of them paid particular attention to the structure-, component-, and composition-dependent electrochemical performance between the electronic structure and active sites of materials and their OER performance as well as the underlying catalytic mechanism explanations. In this review, we present the impressive progress made in the field of low-dimensional Co-based catalysts related to expanding active sites and modulating the electronic structure to improve OER performance. First, the fundamental aspects of electrochemical OERs are introduced. Second, the dimensional control for low Co-based NMs is introduced to increase the active sites for illustrating their electrochemical performance. Third, the role of vacancies such as cationic vacancies and anionic vacancies for Co-based NM is stated for discussing the impact on the electronic structure for their OER activity. Fourth, exposing different crystal directions/facets or designing amorphous structures is proposed to reveal the relationship between two factors and the OER performance. Fifth, constructing hybrid structures of Co-based catalysts is also present to discuss the synergistic effects between different phases. Finally, a brief conclusion about recent progress achieved in oxygen evolution on Co-based NMs, as well as an outlook on future research challenges from the viewpoint of rational design of highly efficient electrocatalysts, is also presented.
2 EVALUATION INDEX FOR OER
The following parameters of catalyst are widely accepted for evaluating the catalytic performance of OER and the basic meanings and importance of these parameters are clarified in detail.
2.1 Overpotential
Overpotential serves as an indicator of catalytic ability, which can be influenced by various factors, such as activity, surface structure, defects, active sites, and catalytic kinetics of the catalysts. A lower overpotential implies better catalytic performance as it requires less extra energy to drive the OER.
2.2 Tafel slope
Tafel slope is affected by various factors, including the properties of the electrode material, the state of the electrode surface, the composition of the electrolyte, and the temperature. A smaller Tafel slope indicates a faster kinetic process since the Tafel slope is inversely proportional to the electron transfer number. The most commonly used method for extracting Tafel values is fitting potentiodynamic polarization curves of LSV. However, this method may not be entirely accurate, as Tafel slope analysis should be based on steady-state responses.77 Therefore, it is strongly recommended to fit true steady-state polarization curves to calculate the Tafel values by using galvanostatic responses to eliminate the effects of double-layer charging and the catalyst's self-oxidation current contributions.78
2.3 Mass activity and specific activity
Mass activity, which quantifies a material's catalytic performance normalized to its mass, is often measured as current density per catalyst mass (mA mg−1). In contrast, specific activity represents the catalytic activity normalized to the electrochemically specific surface area of the catalysts, measured as current density per surface area (mA cm−2). Two primary methods typically determine the specific surface area by using nitrogen adsorption and desorption curves through the Brunauer–Emmett–Teller method to determine the surface area, or by using electrochemical active surface area (ECSA) determined by cyclic voltammetry curves with different scan rates. Comparison of these factors helps discern the dominant influence of mass or surface area on catalysts' efficiency, distinguishing between catalysts with similar mass but different surface areas. This comparison shows guidance in catalysts' design, evaluation, and selection for diverse electrochemical reactions.
2.4 Turnover frequency (TOF)
In practice, TOF is often calculated by using the total molar number of catalyst as that of active sites (that is much smaller than the former), so calculated TOF value is often lower than the real one. TOF is a fair comparison factor to compare catalysts with different structures or compositions regardless of their mass or surface area since it normalizes the reaction rate to the number of active sites.
2.5 Double layer capacitance (Cdl) and ECSA
2.6 Stability
Durability is a critical factor in evaluating catalysts because practical applications demand both high activity and long-term stability. The catalytic stability can be evaluated by three aspects. (i) Catalytic stability: Monitoring the current or voltage changes of a catalyst at specific values by chronopotentiometry or chronoamperometry methods can provide insights into its stability and catalytic ability. The higher ratio of current or the potential at the initial and the final points shows the higher catalytic stability of the catalysts. (ii) Adhesion stability: In typical electrochemical evaluations, the catalyst is applied onto a conductive substrate, such as glassy carbon, carbon paper/cloth, and Ni/Fe/Cu foam. During the OER, numerous bubbles are generated, creating stress that can lead to cracking or detachment of the catalyst film from the electrode surface. Therefore, the catalysts should possess strong adhesion ability to the electrode surface, ensuring their stability and longevity for sustained testing. (iii) Morphology, crystal structure, and elemental composition stability. During OER, catalysts' surfaces often undergo restructuring, forming thin oxyhydroxide layers. These layers are typically the active sites where the catalytic reaction occurs. Tracking alterations in the surface morphology, including the formation, growth, or dissolution of these oxyhydroxide layers, is crucial for understanding the catalyst's stability and performance over time. The crystal structure of the catalysts can significantly impact their catalytic performance. Phase transformations, lattice strain, or defects within the crystal lattice can affect the active sites and overall activity. In an alkaline environment, metals used as catalysts may undergo corrosion or leaching processes, leading to changes in their composition, which may significantly impact their stability and performance. Corrosion and leaching may lead to a decrease/redistribution in the effective surface area available for catalysis. Therefore, understanding the morphology, crystal structure, and elemental composition stability is crucial for developing durable and efficient catalysts suitable for long-term operation.
2.7 Faraday efficiency
In general, two kinds of electrochemical reaction processes involving Faraday processes and non-Faraday processes occur at the electrode. The Faraday process involves the charge/electron transfer at the electrode–electrolyte interface, resulting in an oxidation/reduction reaction, while for the non-Faraday processes, due to unfavored thermodynamics or kinetics, no charge-transfer reaction occurs. Faraday efficiency (FE) is a crucial parameter to provide insights into the overall reaction effectiveness and selectivity of the electrochemical system in converting electrical energy into chemical products. FE with 100% indicates that all the electrons involved in the electrochemical reaction were used to produce the target product, without any side reactions or losses. In fact, FE is always lower than 100%, implying that some of the electrons are used in unintended reactions, resulting in a reduced yield of the target product.
3 ACTIVE SITES EXPANDING AND ELECTRONIC MODULATION
3.1 Dimensional regulation
Nanostructured and low-dimensional Co-based materials offer several significant advantages of increased surface area,80 enhanced mass transport,81 modified electronic structure,82 improved charge transfer,83 and tailored catalytic sites, which are desired catalysts for OER. Nanostructured materials have a high surface-area-to-volume ratio, providing more exposed active sites for catalytic reactions. Quantum confinement effects in NMs can modulate the electron density of states, electronic band structure, and work function, influencing the adsorption ability of the reactants and accelerating the charge transfer during the OER process.84 The ultrasmall size of nanostructures reduces diffusion distances for reactants and products, enabling faster mass transport and decreasing reactant concentration gradients. The unique surface atoms of nanostructures in their positions, such as edges, corners, and defects, can serve as highly active and selective catalytic sites to boost OER performance. The nanostructured catalysts can be classified into zero-dimensional (0D), including nanoparticles and quantum dots, one-dimensional (1D), including nanowires (NWs), nanotubes, and nanorods, two-dimensional (2D) NSs, and three-dimensional (3D) materials, including bulk materials, porous materials, and nanocomposites. The dimensional modulation of Co-based catalysts has been an effective strategy to improve the OER performance.
3.1.1 Design 0D Co-based catalysts
0D NMs, with their ultrasmall nanometer size, exhibit the largest specific surface area among NMs of different dimensions, leading to the exposure of the largest catalytically active surface area. The size of Co-based nanoparticles can influence the OER catalytic activity. Esswein et al.85 investigated the OER properties of cubic Co3O4 nanoparticles with varying average particle sizes of 5.9, 21.1, and 46.9 nm prepared by varying the water content in an ethanol/water solvent system and heating at 150°C in an autoclave. As the particle size decreased, the overpotential at 10 mAcm−2 also decreased from 382 to 328 mV. The smallest size of Co3O4 exhibited the best OER performance, which is attributed to its largest specific surface area and increased number of active sites. Atomically precise dispersion of Co clusters is an effective strategy to maximize exposure to the active sites. Zhou et al. designed highly dispersed Co clusters decorated onto nitrogen-doped carbon nanotubes showing the OER overpotential of 270 mV at 10 mA cm−2 due to the large active area, good electrical conductivity, and more exposed active sites.86 Co quantum dots have quantum confinement effects and can be efficient OER catalysts. Govindhan et al. reported the Co quantum dots (about 3.2 nm) uniformly dispersed on reduced graphene oxide (GO) as efficient OER electrocatalysts.87 Such electrocatalysts afforded a mass activity of 1250 Ag−1 at 1.6 V versus RHE, a small Tafel slope of 37 mV dec−1, and a TOF value of 0.188 s−1 in 0.1M KOH due to the abundant active sites of the fine dispersed Co quantum dots and enhanced electron transfer process.
3.1.2 Design 1D Co-based catalysts
1D Co-based NMs possess a high aspect ratio (length-to-diameter ratio), resulting in an extended surface area along the elongated axis, which increases exposed active sites, enhances electron and mass transport, and reduces charge transfer resistance. Based on the special structural characteristics of 1D materials, many researchers have designed 1D Co-based nanocatalysts for OER.
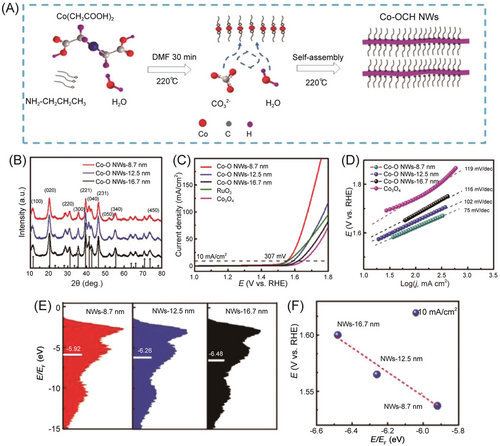
The structure parameters of 1D materials can significantly influence the OER electrocatalytic activity. For example, He et al.88 investigated the relationship between the diameters of Co carbonate NWs (Co-O-H NWs) and OER performance. The authors used the liquid-phase synthesis method to prepare three kinds of Co-O-H NWs with a diameter ranging from 8.5 to 16.5 nm. As shown in Figure 3, the prepared Co-O-H NWs exhibited size-dependent electrocatalytic OER activity under alkaline conditions. The thinnest Co-O-H NWs (8.5 nm) exhibited superior OER activity compared to those of 12.7 and 16.5 nm. The outstanding performance of the thinnest Co-O-H NWs (8.5 nm) could be mainly attributed to the increased active site number, enhanced oxygen vacancy content, and the optimal adsorption energy of the oxygen-containing functional group.
1D Co-based catalysts with different morphology have also been investigated. For instance, Miao et al. prepared high-quality Co3O4 porous nanotubes with abundant active atoms and effective mass transfer channels; therefore, the Co3O4 nanotube shows high OER activity and good durability.89 Kim and colleagues synthesized CoNy@CoOx core–shell nanorod hybrid catalysts combined with N-doped carbon nanofibers, which displayed high OER performance and durable stability in alkaline media.90 The good OER performance was attributed to the fast charge transfer at the metallic Co4N core part. Wan et al. synthesized a peculiar Co-based phosphate NaCo4(PO4)3 nanoribbon, in which all Co atoms were in rare 5-coordinations.91 Thanks to the combined merits of the unique Co coordination and intrinsic half-metallicity, NaCo4(PO4)3 nanoribbons displayed high efficiency for OER in a neutral electrolyte.
3.1.3 Design 2D Co-based catalysts
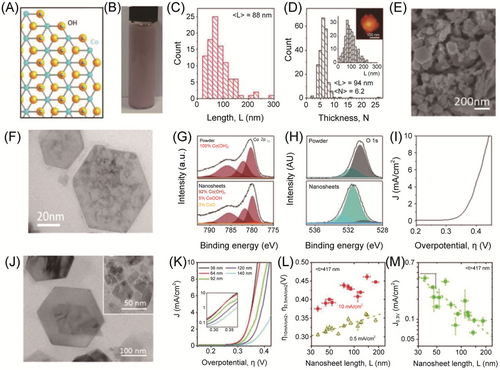
2D NMs possess an inherently high surface area per unit mass due to their ultrathin thickness with only a single or few-atom thickness, allowing for facile exposure to active catalytic sites. The surface area of NSs is adjustable by modulating their diameter and thickness, thus affecting the OER catalytic ability. McAteer et al. prepared Co(OH)2 NSs with adjustable diameters by a liquid exfoliated method as the OER catalysts.92 As shown in Figure 4, the smallest Co(OH)2 NSs displayed higher OER performance than larger NSs. Additionally, the hybridization of 2D NSs with 10 wt% 1D nanotubes exhibited the best OER performance with a low overpotential of 235 mV at 50 mA cm−2, enhanced conductivity, and better stability.
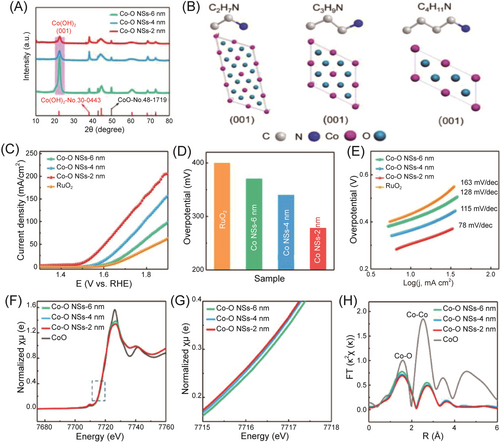
Thickness regulation of 2D NSs is a promising strategy for improving catalytic performance. However, achieving precise control, especially in ultrathin limits with only a few atomic layers, remains a significant challenge. He et al. made progress in precisely modulating the thickness of 2D NSs.60 By employing alkylamine as ligands and adjusting the vertical coordination capacity, they successfully synthesized ultrathin cobaltous hydroxide and cobaltous oxide hybrid (Co(OH)2-CoO) NSs (CO-O NSs) with an adjustable thickness of 2–6 nm (5–13 atomic layers). As shown in Figure 5, ultrathin CO-O NSs with a thickness of 2 nm (Co-O NS-2 nm) showed the best OER performance, which is attributed to their increased active sites, superior adsorption strength, and highest electrical conductivity.
3.1.4 Design 3D Co-based array
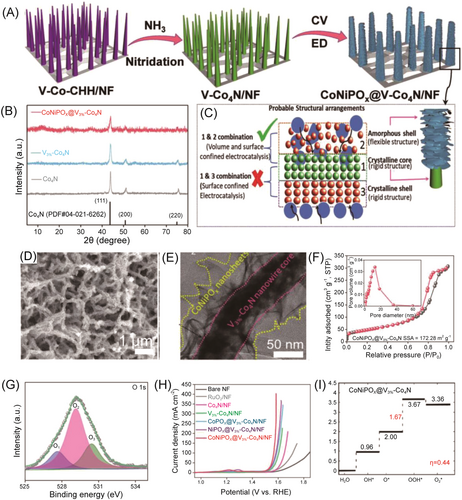
Compared to other dimensional NMs, 3D Co-based nanoarrays combine the advantageous characteristics of good electron transport of 1D NMs and the presence of numerous active sites of 2D NMs, resulting in improved charge and mass transfer processes and enhanced stability, making them potentially as efficient OER catalysts. For example, Chen et al. designed and synthesized a 3D heterojunction array (CoNiPOx@V3%-Co4N) by combining 1D V-doped Co4N crystals and amorphous ultrathin 2D CoNiPOx NSs directly grown on Ni foam (Figure 6). Such a 3D array structure exhibited high surface area and mesoporous properties; it served as both anode and cathode catalysts for the water-splitting cell, achieving a low potential of 1.52 V at 10 mA cm−2, which was much lower than that of an electrolytic cell consisting of 20% Pt/C (−)//RuO2 (+).93 The excellent electrochemical performance of CoNiPOx@V3%-Co4N can be attributed to its increased active sites, enhanced electrochemical area, and low charge transfer resistance. In addition, Zhu et al. developed a template method for preparing hierarchical hollow 3D Co3O4 microtube arrays supported on conductive Ni foam.94 The Co3O4 microtube arrays exhibited high activity and good stability (in both OER and HER) that are comparable to those of Pt/C and IrO2/C catalysts, which is attributed to enhanced mass transfer ability and facilitated electron migration.
In summary, rationally controlling dimensions and adjusting the geometric parameters for Co-based catalysts can enhance OER performance. Co-based catalysts that enhance the OER performance by adjusting the dimensions are summarized in Table 1.
| Materials | Support | Overpotential (mV) @ 10 mA cm−2 | Tafel slope (mV dec−1) | References | |
|---|---|---|---|---|---|
| 0D | Co3O4 NPs | GC | 328 mV for the size of 5.9 nm | 47 ± 7 (vs. BET) | [85] |
| 363 mV for the size of 21.1 nm | - | [85] | |||
| 382 mV for the size of 46.9 nm | - | [85] | |||
| Co NPs and Co nanoclusters | CNTs | 270 | 79 | [86] | |
| Co QDs | rGO | / | 37 | [87] | |
| 1D | Co-O-H NWs | GC | 307 mV for the diameter of 8.7 nm | 75 | [88] |
| 330 mV for the diameter of 12.5 nm | 102 | [88] | |||
| 377 mV for the diameter of 16.7 nm | 116 | [88] | |||
| Co3O4 nanotubes | GC | 323 | 56 | [89] | |
| CoOx@CoNy NRs | NCNF | 460 | 85.6 | [90] | |
| NaCo4(PO4)3 nanoribbons | GC | 373 mV @ 1 mA cm−2 | 121 | [91] | |
| 2D | Co(OH)2 NSs | GC | 235 mV @ 50 mA cm−2 | / | [92] |
| Co(OH)2-CoO NSs | GC | 278 mV for the thickness of 2 nm | 78 | [60] | |
| 340 mV for the thickness of 4 nm | 115 | [60] | |||
| 370 mV for the thickness of 6 nm | 128 | [60] | |||
| 3D | CoNiPOx@V3%-Co4N | Ni foam | 270 | 54.66 | [93] |
| 3D Co3O4 microtube arrays | Ni foam | 360 mV @ 150 mA cm−2 | 84 | [94] |
- Abbreviations: 0D, zero-dimensional; 1D, one-dimensional; 2D, two-dimensional; 3D, three-dimensional; BET, Brunauer–Emmett–Teller; CNT, carbon nanotubes; GC, glassy carbon; NCNF, N-doped carbon nanofibers; NP, nanoparticles; NR, nanorods; NS, nanosheets; NW, nanowires; OER, oxygen evolution reaction; QD, quantum dot; rGO, reduced graphene oxide.
3.2 Vacancy adjustment
Defect engineering is one of the effective approaches to enhance the catalyst activity.95-97 Vacancies, which refer to missing atoms in the crystal lattice of a material, can enhance the OER performance by tailoring the metallic electronic structure and adsorption/desorption properties of the oxygen intermediates.98-100 Advanced techniques, such as synchrotron radiation X-ray absorption spectroscopy, positron annihilation lifetime spectroscopy, electron paramagnetic resonance spectroscopy, electron energy loss spectroscopy, and so forth, have been increasingly utilized to study the defects, offering in-depth and detailed insights into electrocatalysts at the nanoscale or subnanoscale levels.101, 102 Anion vacancy and cation vacancy were all investigated to be efficient to enhance the OER performance.
3.2.1 Anion vacancies
Oxygen vacancies
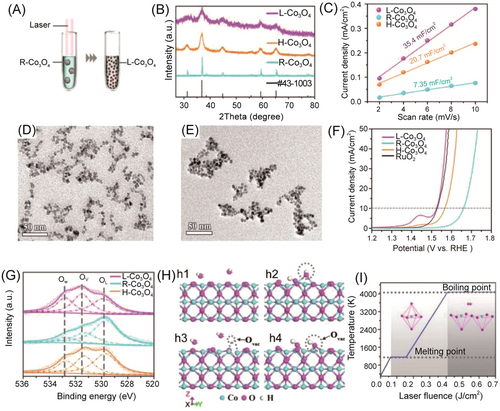
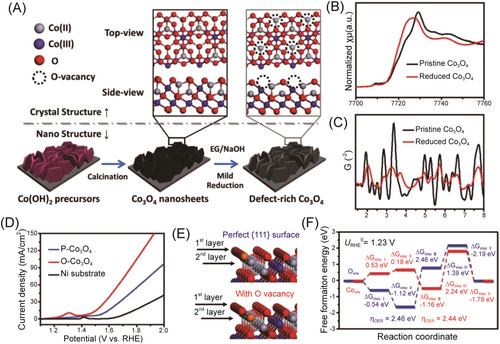
Oxygen vacancies are prevalent anionic vacancies in transition metal oxides, characterized by low formation energy, which can significantly influence the physical and chemical properties of the oxides.96 The presence of oxygen vacancies plays a crucial role in adjusting the band gap of semiconductors and altering the electron distribution on the surface of the materials, thus having the potential to increase the conductivity and fine-tune the adsorption ability between the catalysts and the reactants/intermediates/products.103, 104 For instance, Xu et al. synthesized Co3O4 NSs engineered with oxygen vacancies by thermal annealing and Ar plasma etching treatment.105 The resulting Co3O4 nanoparticles with oxygen vacancies exhibited superior electrocatalytic activity for the OER compared to the original Co3O4. Through comprehensive characterization analysis, it was found that the oxygen vacancies led to a higher ratio of Co3+/Co2+ in defected Co3O4, thereby enhancing the electronic conductivity of the material and generating more active sites, including the defect sites. Zhou et al. successfully synthesized Co3O4 nanoparticles with abundant oxygen vacancies using the laser irradiation method.106 As shown in Figure 7, the defected catalyst showed good OER performance under alkaline conditions, surpassing the precious commercial catalysts of RuO2. Both experimental characterization and theoretical calculation demonstrated that the oxygen vacancies induced in the Co3O4 nanoparticles facilitated the adsorption of water molecules and improved the electrical conductivity of the material, thus significantly enhancing its electrocatalytic OER performance.
The single-crystal ultrathin Co3O4 NSs with O-terminal (111) surface with oxygen defects were prepared using a mild solvothermal reduction method by employing ethylene glycol as the solvent under alkaline conditions.107 As shown in Figure 8, the defect-rich Co3O4 NSs exhibited a remarkably low overpotential of 220 mV for OER and a Tafel slope of 49.1 mV dec−1. Notably, the introduction of oxygen vacancies exposed the second layer of Co metal centers in the Co3O4 NSs, reducing the OER activation energy to 2.26 eV.
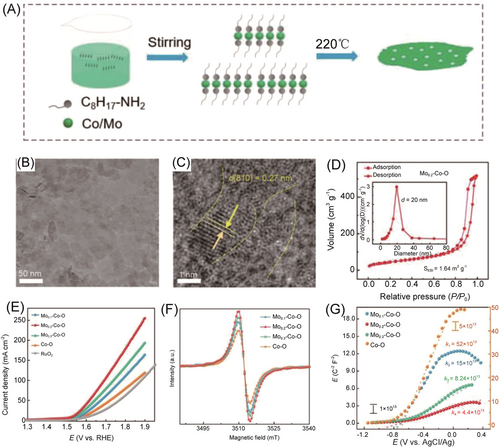
Furthermore, mesoporous Mox-Co-O hybrid NSs composed of crystalline phase Mo4O11 and amorphous phase Co hydroxide were prepared with octylamine as a ligand under a simple one-pot ethylene glycol solvothermal route.108 As shown in Figure 9, the prepared Mo0.2-Co-O NSs exhibited excellent OER activity with the lowest overpotential of 276 mV at 10 mA cm−2 in 1 M KOH, which was contributed to the suitable d-band center and the highest concentration of oxygen vacancies. In addition, Chen and colleagues used CoCl2/K3Co(CN)6 cyanogen gel as the reaction precursor to directly synthesize ultrathin nanonetwork Co3O4 NMs (Co-UNMs) by a one-step method without corrosion.109 The prepared Co-UNMs had a thickness of 1.5 nm with abundant pores, contributing to a high specific surface area and a large number of defects. Consequently, the Co-UNMs demonstrated improved OER activity in alkaline media with a low overpotential of 307 mV at 10 mA cm−2 and a Tafel slope of 76 mV dec−1.
Selenium vacancies
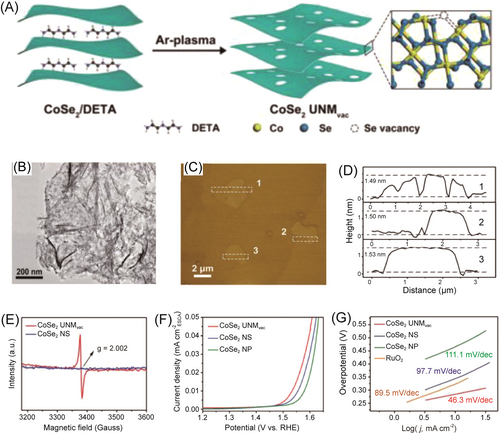
Besides generating oxygen vacancies, constructing selenium vacancies is also an effective strategy for metal selenides to promote OER performance. CoSe2, with its suitable electron configuration and inherent metal behavior, holds promise as an OER electrocatalyst.110, 111 However, the limited active sites in CoSe2 nanomaterials inhibit their electrocatalytic OER performance. To address this, Zhang et al. obtained a grid of CoSe2 with abundant selenium vacancies (CoSe2 UNMvac) through diethylenetriamine (DETA) plasma treatment preparation.112 As shown in Figure 10, CoSe2 UNMvac showed good OER performance with an overpotential of 300 mV and a Tafel slope of 46.3 mV dec−1, which can be attributed to the increased active sites on the surface.
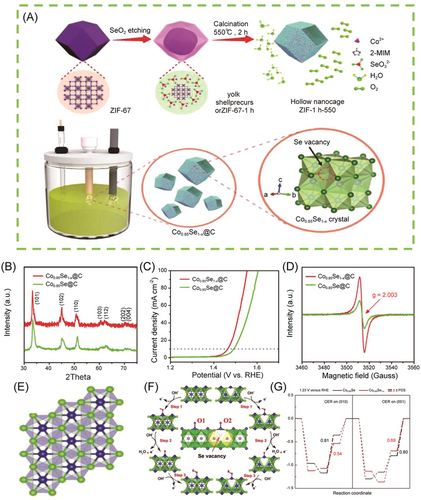
Furthermore, a series of carbon-supported 3D transition metal selenides MmSen (Co0.85Se1−x, NiSe2−x, FeSe2−x) with selenium vacancies was synthesized using a selenite-assisted etching method by Zhang et al. to investigate the role of the selenium vacancies.113 Among them, Co0.85Se1−x@C nanocages exhibited excellent electrochemical water-splitting characteristics, displaying an overpotential of 231 mV at 10 mA cm−2 for OER (Figure 11). Density-functional theory (DFT) calculations revealed that the presence of abundant selenium vacancies reduced the intermediate reduction barrier and improved the catalyst's conductivity.
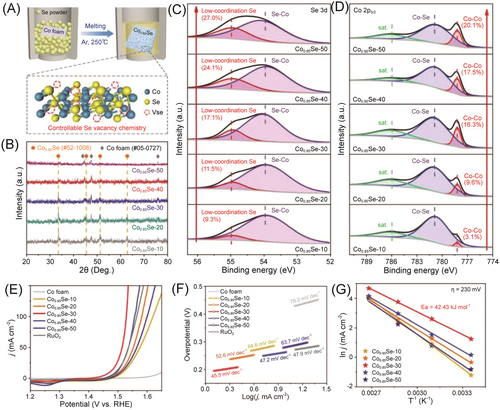
Moreover, the OER performance is highly related to the quantities of the selenium vacancies. For instance, a series of selenium-vacancy-adjustable Co0.85Se NSs was successfully synthesized using a rapid fusion synthesis method with temperature control. Electrochemical experiments revealed that the OER activity of Co0.85Se was dependent on the selenium vacancy content. Among them, the Co0.85Se-30 catalyst displayed excellent activity with an overpotential of 243 mV at 10 mA cm−2 and a Tafel slope of 45.5 mV dec−1 (Figure 12). Further electrochemical analysis indicated that the abundant selenium vacancies in Co0.85Se-30 enhanced the intrinsic activity of Co active centers (jCo = 6.49 A g−1 at η = 270 mV) and lowered the activation energy (42.43 kJ mol−1).
3.2.2 Cation vacancies
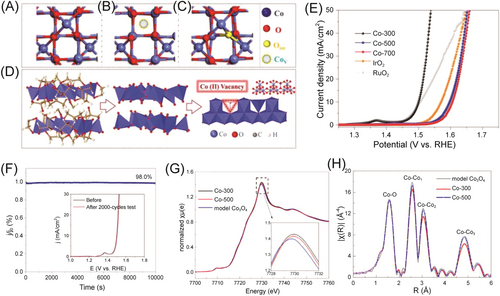
Most of the anion vacancies, such as oxygen vacancies or selenium vacancies, are unstable and susceptible in an oxygen environment; they get filled with oxygen atoms from the surrounding atmosphere or electrolyte, reversing the defect and potentially affecting their stability.106 Therefore, constructing the vacancies for the metal atoms is another good option. Several commonly used methods have been developed for generating cation vacancies and tailoring their content in transition metal oxides, such as selective removal of constituent cation by acid etching,115, 116 organic molecules assist,117, 120 thermal annealing,118 and plasma etching.119 For example, Co3−xO4 nanoparticles with Co defects were prepared with thermal calcination, in which metal defect was easily formed in a layered structure of oxide crystals. In addition, X-ray absorption fine structure and positron annihillation lifetime spectra analyses revealed the presence of abundant Co vacancies and a twisted structure in the Co3−xO4 (Figure 13).120 DFT calculations indicated that these metal defects induced significant electron delocalization, thereby enhancing the carrier's transportation along the defect conductive channel and facilitating the adsorption/activation of water on the catalysts' surface. Compared with ordinary Co3O4 (376 mV), IrO2 (340 mV), and RuO2 (276 mV), Co3−xO4 with Co defects showed a better OER performance with the overpotential of 268 mV at 10 mA cm−2.
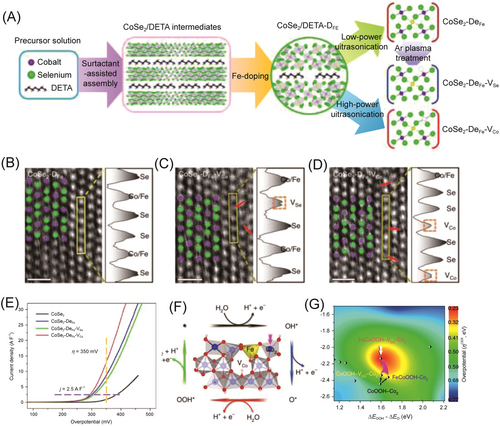
Likewise, ultrathin CoSe2 nanoribbons doped with Fe and Co vacancies were synthesized to be efficient OER catalysts, in which Co vacancies were extracted by DETA molecules during the exfoliation of CoSe2/DETA under high ultrasonic power.117 As shown in Figure 14, in-depth characterization and DFT calculations identified that the most active centers were located at Co2 sites adjacent to the Fe and Co vacancies, making optimized electronic states of Co2 close to the optimal value, which resulted in a decrease in the binding energy of OH*.
The recent progress in enhancing the OER performance by introducing vacancies in Co-based electrocatalysts is summarized in Table 2. Overall, vacancies are unique defect sites where the coordination environment and electronic structure differ from the perfect lattice. These defect sites can provide more accessible locations for OER intermediates to adsorb and react, promoting catalytic activity. The altered electronic structure around vacancies can influence the adsorption energy of OER intermediates (such as adsorbed oxygen atoms or hydroxyl species). Moreover, vacancies can modulate the charge distribution and electronic conductivity of the catalysts, which can facilitate more efficient charge transfer during the OER, reducing energy barriers and improving overall electrocatalytic performance. However, careful consideration is needed to balance the benefits of enhanced catalytic activity with potential stability issues that may arise from excessive defect formation. The controlled introduction and manipulation of vacancies, along with a thorough understanding of their effects, are crucial for OER and other electrocatalytic processes.
| Vacancies | Materials | Support | Overpotential (mV) @ 10 mA cm−2 | Tafel slope (mV dec−1) | References |
|---|---|---|---|---|---|
| Oxygen vacancies | Co3O4 NSs | Ti foil | 540 | 234 | [105] |
| Co3O4NSs with vacancies | Ti foil | 300 | 68 | [105] | |
| L-Co3O4 NPs with vacancies | Carbon fiber | 294 | 74 | [106] | |
| H-Co3O4 | Carbon fiber | 336 | 88 | [106] | |
| R-Co3O4 NPs | Carbon fiber | 430 | 92 | [106] | |
| O-Co3O4 NSs with vacancies | Ni foam | 220 @ 0.1 mA cm−2 | 49.1 | [107] | |
| P-Co3O4 NSs | Ni foam | 300 @ 0.1 mA cm−2 | 72.3 | [107] | |
| Mo0.2-Co-O NSs with the highest vacancies | GC | 276 | 63 | [108] | |
| Mo0.3-Co-O NSs with moderate vacancies | GC | 303 | 76 | [108] | |
| Mo0.1-Co-O NSs with low vacancies | GC | 321 | 92 | [108] | |
| Co-O NSs with the lowest vacancies | GC | 340 | 101 | [108] | |
| Co-O NMs with defects | GC | 340 @ 0.1 mA cm−2 | 76 | [109] | |
| Co NSs | GC | 363 @ 0.1 mA cm−2 | 95 | [109] | |
| Selenium vacancies | CoSe2 NMs with Se vacancies | GC | 284 | 46.3 | [112] |
| CoSe2 NSs | GC | 343 | 97.7 | [112] | |
| CoSe2 NPs | GC | 466 | 111.1 | [112] | |
| Vacancy-poor Co0.85Se | GC | 291 | 75 | [113] | |
| Co0.85Se1−x@C nanocages with Se vacancies | GC | 231 | 57 | [113] | |
| Vacancy-poor NiSe2 | GC | 270 | 110 | [113] | |
| NiSe2−x@C microspheres with Se vacancies | GC | 242 | 92 | [113] | |
| Vacancy-poor FeSe2 | GC | 296 | 54 | [113] | |
| FeSe2−x @ C nanoneedles with Se vacancies | GC | 262 | 42 | [113] | |
| Co0.85Se NPs with Se vacancies | Co foam | 243 | 45.5 | [114] | |
| Co defects | Co3−xO4–VCo | GC | 268 | 38.2 | [120] |
| Co3O4 | GC | 376 | / | [120] | |
| CoSe2–DFe–VCo | GC | 294.2 @ 2.5 A F−1 | 53.5 | [117] | |
| CoSe2–DFe | GC | 300.4 @ 2 A F−1 | 66 | [117] | |
| CoSe2–VCo | GC | 307.3 @ 2.5 A F−1 | 70.5 | [117] | |
| CoSe2 | GC | 385.7 @ 2.5 A F−1 | 72.6 | [117] |
- Abbreviations: GC, glassy carbon; NM, nanomaterial; NP, nanoparticles; NS, nanosheets; NW, nanowires; OER, oxygen evolution reaction.
3.3 Controlling crystal structure
3.3.1 Exposing different crystal facets
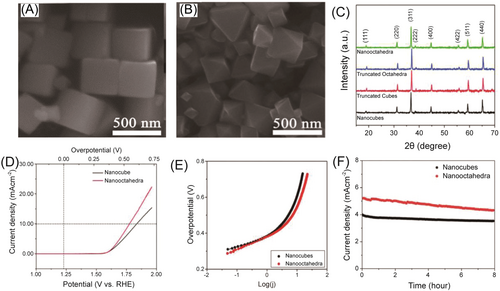
Designing different crystal directions or facets in electrocatalysts can significantly enhance OER performance.121-123 The arrangement of atoms and crystallographic planes on a specific facet can lead to variations in the electronic structure of the material, affecting the adsorption energies of reaction intermediates and potentially accelerating reaction kinetics.124, 125 For instance, well-defined cubic and octahedral Co3O4 nanoparticles with the predominantly exposed (100) and (111) were synthesized by Koel and colleagues.68 As shown in Figure 15, the cubic Co3O4 with exposing (100) facets showed a higher OER activity and stability than octahedral Co3O4 with exposing (111) facets, which demonstrates the importance of surface orientation. Besides, the adjustment of exposure crystal facets could also modulate the spin state of Co3+ in Co-based perovskite single-crystal thin film (PrBaCo2O6), thereby optimizing the OER performance. This study concluded that PrBaCo2O6(100) thin film had stronger adsorption with the oxygen intermediate compared with those of PrBaCo2O6(110) and PrBaCo2O6(111), which is attributed to the hybridization between the Co 3d orbit and O 2p orbit for facilitating a faster electron transfer.126 Moreover, CoMoO4 nanorods were prepared with the mainly exposed facets of (100) and CoMoO4 NSs with the mainly exposed facets of (010). Although CoMoO4 NSs had a larger surface area than CoMoO4 nanorods, CoMoO4 nanorods showed a better OER performance due to the (100) facets with a higher surface energy.127
3.3.2 Synthetic amorphous structure
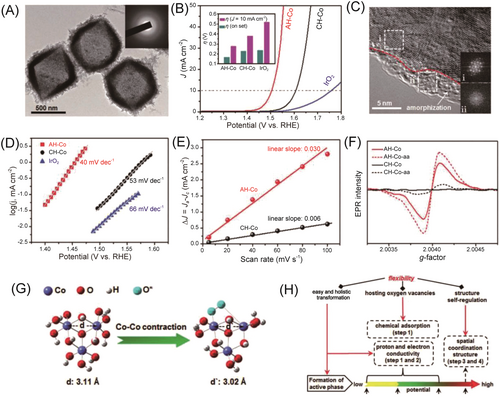
Amorphous catalysts with a lack of long-range order properties and a unique electronic structure make them promising catalysts for enhancing OER performance. For instance, Risch et al. concluded that amorphous CoOx undergoes a chemical oxidation state change, which enables O–O bond formation and promotes the delivery of dioxygen molecules.126 Furthermore, a novel highly dispersed amorphous CoOx was synthesized using air dielectric barrier discharge plasma. Compared to the original biopolymer and Co complex, the amorphous CoOx clusters exhibited a lower overpotential of 290 mV at 10 mAcm−2 for OER, and the excellent electrocatalytic OER activity was attributed to the unsaturated catalytic sites clusters on the amorphous CoOx.128 Moreover, an amorphous cophosphate hydroxide nanocage was prepared and synthesized by Liu et al.,129 and it also exhibited excellent OER performance, which is attributed to the presence of certain oxygen vacancies and structural self-regulation during the OER process (Figure 16). Besides, mixed oxides or hydroxides of Co and other transition metals, such as cobalt iron, cobalt nickel, and other crystalline and amorphous hybrids, also exhibit good performance in electrocatalytic OER.61, 130, 131
The recent progress in enhancing the OER performance by phase modulation by either adjusting the facets of crystals or synthesizing the amorphous structure of Co-based electrocatalysts is summarized in Table 3. In summary, designing different exposed crystal directions or facets or amorphous catalysts is a feasible strategy to tune the electrocatalyst's properties, such as electronic structure and active site distribution, thereby tailoring OER activity.
| Materials | Regulating strategies | Electrochemical performance | Tafel slope (mV dec−1) | |
|---|---|---|---|---|
| Co3O4 nanocubes | Expose (100) surfaces | 15.4 mA cm−2 @ 1.96 V versus RHE | 59 | [68] |
| Co3O4 nanooctahedra | Expose (111) surfaces | 22.2 mA cm−2 @ 1.96 V versus RHE | 67 | [68] |
| PrBaCo2O6 thin film | Expose (100) surfaces | 0.24 mA cm−2 @ 1.6 V versus RHE normalized for ECSA | 92 | [127] |
| PrBaCo2O6 thin film | Expose (111) surfaces | 0.08 mA cm−2 @ 1.6 V versus RHE normalized for ECSA | 159 | [127] |
| PrBaCo2O7 thin film | Expose (110) surfaces | 0.12 mA cm−2 @ 1.6 V versus RHE normalized for ECSA | 124 | [127] |
| CoMoO4 nanorods | Expose (100) surfaces | 8.93 mA cm−2 @ 1.78 V versus RHE normalized for ECSA | 72 | [132] |
| CoMoO4 nanosheets | Expose (010) surfaces | 1.56 mA cm−2 @ 1.78 V versus RHE normalized for ECSA | 98 | [132] |
| Co(OH)2 | Crystallization | 380 mV @ 10 mA cm−2 | 53 | [129] |
| Co(OH)2 nanocages | Amorphous | 280 mV @ 10 mA cm−2 | 40 | [129] |
| CoOx | Amorphous | 290 mV @ 10 mA cm−2 | 47 | [128] |
| Ni0.8Co0.2O-RT | Crystalline–amorphous | 320 mV @ 10 mA cm−2 | / | [61] |
| CoFexOy-SO4 | Crystalline–amorphous | 268 mV @ 10 mA cm−2 | 46.5 | [130] |
- Abbreviations: ECSA, electrochemical active surface area; NC, nanocarbon; OER, oxygen evolution reaction; RHE, reversible hydrogen electrode.
3.4 Constructing composite and hybrid structures
3.4.1 Constructing multiphase or hybrid Co-based catalysts
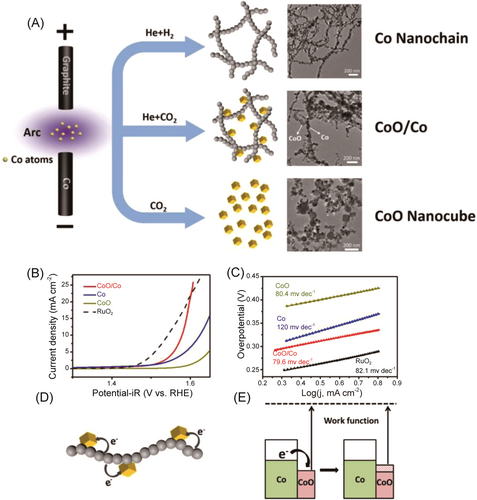
Constructing a multiphase catalyst for OER can be a promising approach to overcome the limitations of single-phase materials. Different phases may have complementary electronic structures and catalytic properties, enabling optimized activity for various OER reaction intermediates. Multiphase catalysts provide more active sites, facilitating faster kinetics and reducing overpotential. The synergistic effects between different phases can enhance the overall catalytic activity beyond the single phase. For instance, three types of samples, Co nanochains, CoO nanocubes, and mixture phase containing these two nanostructures were prepared by modulating the gas atmosphere during the synthesis process by Yuan et al.133 Among them, the mixture phases showed the best OER activity with an overpotential of 350 mV at 10 mA cm−2, which can be attributed to the synergies effect between Co and CoO and the electron transfer at the interface from Co to CoO, thereby changing the work function of the CoO (Figure 17).
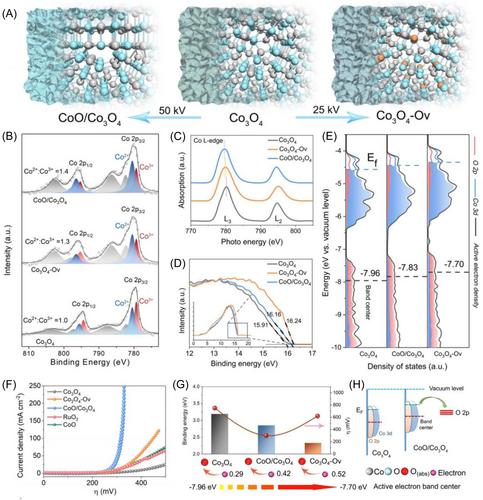
Constructing heterojunction structures is a versatile approach to enhancing catalytic performance by modulating the interface electronic states. For instance, CoO/Co3O4 heterojunction catalysts showed a better OER performance than that of the CoO and Co3O4, with an overpotential of 260 mV at 10 mA cm−2 and a Tafel slope of 54 mV dec−1. As shown in Figure 18, the center of the surface-active electron density band of Co3O4 in the hybrid structure had shifted upward, resulting in a significant increase in the absorption capacity of oxygen-containing radical groups.134
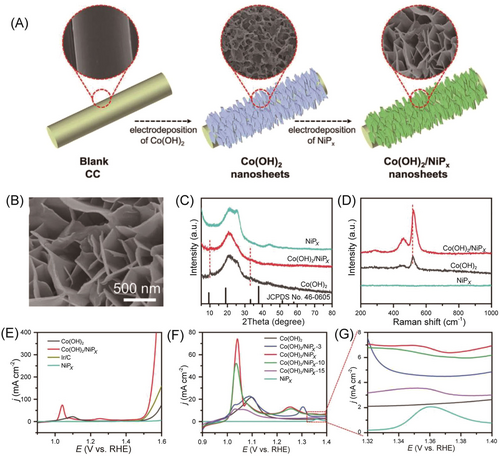
The heterojunction catalysts display enormous development prospects, in which electrons can be rearranged on heterostructure interfaces to promote active sites and the reaction kinetics.37, 135 The heterojunction catalysts often show a better performance for OER than single-component catalysts. For instance, the heterojunction in the Co/CoSe@NC can tune the Fermi level and charge redistribution to accelerate the charge transfer, which displays an efficient OER activity.37 Besides, the interface electronic structure of the Co(OH)2/NiPx heterojunction can be modulated by changing the loading of NiPx.136 The best-loaded Co(OH)2/NiPx heterogeneous NSs had an overpotential of 236 mV at a current density of 10 mA cm−2, better than that of Co(OH)2 (292 mV) and the reference Ir/C (262 mV) (Figure 19). The strong electronic interaction between Co(OH)2 and NiPx was conducive to the formation of high-valence Co centers, which greatly promoted the electron and mass transfer, and thus also significantly improved the electrocatalytic OER performance.
3.4.2 Incorporating Co-based catalysts onto suitable supports
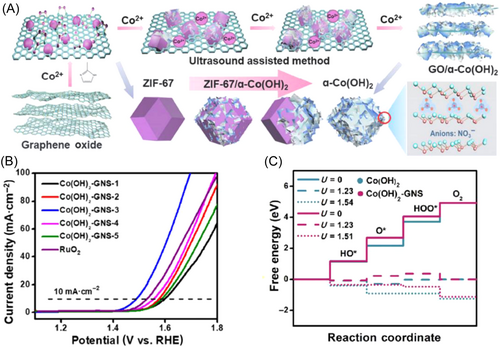
Incorporating Co-based catalysts onto suitable supports is a common strategy in catalysis, which can anchor, isolate, and stabilize the catalysts, preventing the leach/aggregation of active species and maintaining long-term catalytic activity. Additionally, the support material, especially the conductive support, can enhance the charge transfer to enhance the catalytic ability. For example, via an ultrasound-assisted coordination modulation strategy, Huang et al. constructed Co(OH)2/GO composites derived from a sandwich-like metal-organic framework through a one-step self-transformation pathway.137 The dodecahedron ZIF-67 with a tetrahedral coordination of Co2+ automatically transformed into a defection-rich ultrathin-layered Co(OH)2 under the action of the interlayer ion NO3−. The self-transforming α-Co(OH)2/GO NS array altered the coordinated way of Co2+, exposing more metal active sites and thereby improving space utilization within the framework. The optimized Co(OH)2/GO NSs exhibited significant OER catalytic performance with an overpotential of 259 mV at 10 mA cm−2 in alkaline media, better than that of the commercial RuO2 (Figure 20). DFT calculations further investigated the synergistic effect of ultrathin Co(OH)2 NSs with GO, which played important roles in reducing the energy barrier of the intermediate product and greatly improving the electrocatalytic performance.
Incorporating catalysts on the conductive substrate can improve desired catalytic activity, as a result of enhanced conductivity and potential synergistic effects. However, the coordination environment of a catalyst is pivotal in influencing its catalytic behavior. Huang et al. used different mononuclear Co catalysts (CoN4Cl, CoCN3Cl, and CoC4Cl) to incorporate into the graphene matrix to investigate the coordination environment effect on OER. The binding strength for Co=O* became stronger when replacing N with by C. The CoCN3Cl with graphene matrix showed the most suitable binding strength of Co=O* and thus obtained the best OER performance. Besides, they found that different coordination environments of the catalysts led to different rate-determining steps (RDSs) in OER. The RDS for CoCN3Cl and CoN4Cl was Co-OH*-to-Co=O* transition, while the RDS for CoC4Cl was Co=O*-to-Co-OOH* transition.138
The recent progress in enhancing the OER performance by constructing composites and hybrid structures is summarized in Table 4. Constructing composites and hybrid structures of the Co-based catalysts by synthesis of different Co-based phases with complementary catalytic properties or designing heterostructures by combining Co-based catalysts with other materials can create heterointerfaces that modulate the electronic properties and promote efficient charge separation and transfer, thus enhancing the OER performance.
| Materials | Overpotential (mV) @ 10 mA cm−2 | Tafel slope (mV dec−1) | References |
|---|---|---|---|
| CoO/Co hybrid structure | 350 | 76.9 | [121] |
| Co nanochains | 390 | 120 | [133] |
| CoO nanocubes | 440 | 80.4 | [133] |
| CoO | 300 | / | [134] |
| Co3O4 | 410 | 139 | [134] |
| Co3O4 with oxygen vacancies | 300 | 92 | [134] |
| CoO/Co3O4 hybrid structure | 270 | 55 | [134] |
| heterostructured Co(OH)2/NiPx | 220 @ 0.1 mA cm−2 | 52 | [136] |
| Co(OH)2 | 251 @ 0.1 mA cm−2 | 87 | [136] |
| NiPx | >500 | 237 | [136] |
| α-Co(OH)2/GO nanosheet arrays | 259 | 85.9 | [137] |
| Co/NG (Co coordinated with one Cl and four N atoms) | >500 | 178 | [138] |
| Co/G (Co coordinated with one Cl and four C atoms) | 396 | 87 | [138] |
| CoNx/G (Co coordinated with one Cl and four C atoms) | 359 | 81 | [138] |
| Co/CoSe@NC | 335 | 77.6 | [37] |
- Abbreviations: GO, graphene oxide; NC, Schottky junctions encapsulated in carbon; NG, nitrogen doped graphene; OER, oxygen evolution reaction.
4 OUTLOOK AND PERSPECTIVE
The electrochemical water-splitting reaction as a clean and green way to produce hydrogen has obtained much attention. This review summarizes the research progress in Co-based catalysts enhancing OER performance by expanding the active sites or modulating the electronic structure. Special points are focused on the progress in dimensional (size) regulation, defect (vacancy) regulation, phase regulation, and hybrid structure construction. Specifically, 0D–3D Co-based catalysts are investigated to be effective OER catalysts due to their specific dimensional characteristics by expanding the active area. Introducing defects in nanomaterials can modulate the electronic structure, optimize the adsorption/desorption ability of oxygen-containing functional groups, and reduce the Gibbs free energy barrier. Designing different exposed crystal directions or facets or amorphous catalysts can adjust the electronic structure and active site distribution. Constructing composites and hybrid structures of the Co-based catalysts can create heterointerfaces to modulate the electronic properties, and promote efficient charge separation and transportation, thus enhancing the OER performance.
- (1)
The active sites in nanomaterials can be further enlarged. Despite large efforts that have been made to synthesize 0D–3D nanomaterials, these materials still fall short of fully exposing the active sites, thus limiting the electrocatalytic performance. Therefore, nanomaterials with a smaller size, especially only with a single atom layer, are identified to maximize the active sites. Consequently, ongoing research endeavors are directed toward producing subnanometer-sized transition metal catalysts, which can be considered one of the most effective OER catalysts.
- (2)
Ion vacancies emerge as a vital tool for modulating the electronic structure and enhancing intrinsic activity. Anionic vacancies, such as oxygen or selenium vacancies can boost OER performance by increasing the conductivity and optimizing the adsorption/desorption ability. Cationic vacancies, noted for their stability relative to anionic vacancies, tend to induce local structural distortions to affect the charge distribution, leading to lower catalytic energy barriers. However, researchers currently lack precise quantitative characterization of ion vacancies in nanomaterials, which is crucial for revealing the relationship between vacancy quantity and electrocatalytic performance. This underscores the potential significance of future research directions focused on ion vacancy regulation in electrocatalysis.
- (3)
An essential avenue for enhancing catalytic performance involves enhancing conductivity by incorporating highly conductive components, such as composites, conductive carbon powders, and graphene materials. However, the effective integration of these materials presents a significant challenge, as it necessitates a delicate balance between electrocatalytic activity and stability. It is well known that there are fewer nanomaterials suitable for the OER under acidic conditions compared to those designed for alkaline environments. Hence, it is necessary to design the advanced transition metal-based OER catalysts applied in an acidic environment.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (22172063), the Young Taishan Scholars Program (tsqn201812080), the China Scholarship Council (CSC) for scholarship support (202008130132), and the Independent Cultivation Program of Innovation Team of Ji'nan City (2021GXRC052).
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interests.
Biographies

Chuansheng He received his PhD. degree (2021) in Physical Chemistry from Beijing Normal University. He was a postdoctoral fellow in Professor Xiaoqing Huang's group at Xiamen University (2021–2023). Then, he joined the University of Jinan. His research focuses on the design of nanomaterial structures and their electrocatalytic performance.

Linlin Yang received her master's degree in Optical Engineering from Hebei University (2020), China, and as a visiting student, studied at Adelaide University, Australia (2019). Currently, she is a PhD. candidate researching electrocatalysts, fuel cells, and zinc–air batteries at the University of Barcelona, Spain, and the Catalonia Institute for Energy Research, Spain.

Jia Wang is currently a doctoral student at the Graduate School of Integrated Sciences for Life of Hiroshima University under the supervision of Prof. Atsuhiko Ishida. Her research focuses on the studies of carbonylation of CaM kinase phosphatase (CaMKP/PPM1F/POPX2) by polyphenols and its physiological effects.

Tingting Wang received her bachelor's degree in Medical Laboratory Technology in 2019 from Wenzhou Medical University and her master's degree in Pathogeny Biology in 2023 from Wenzhou Medical University, China. She joined Wenzhou Institute, University of Chinese Academy of Sciences as a research assistant in 2023. Her research interests include the pathogenic mechanism of Toxoplasma gondii, the molecular biology of Toxoplasma gondii, and the design and preparation of nanomaterials for electrochemical immunodiagnostic disease studies.

Jian Ju is currently an associate professor at the Wenzhou Institute, University of Chinese Academy of Sciences. He received his PhD. degree in Analytical Chemistry from Northeast Normal University, Changchun, China, in 2013. He then worked as a postdoctoral fellow at Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Nanyang Technological University in Singapore, and Oakland University, USA. His current research interests include the design and synthesis of metal and carbon nanomaterials for electrocatalysis, electroanalysis, biosensors, and accurate diagnosis.

Yizhong Lu is currently a full professor in the School of Materials Science and Engineering, at the University of Jinan. He received his PhD. degree from the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, in 2015. He then worked as a research fellow in the School of Chemical and Biomedical Engineering at NTU. His research interests include the design, synthesis, and electrocatalytic applications of nanomaterials for fuel cells and water splitting.

Wei Chen received his PhD. degree in Electrochemistry from Xiamen University under the direction of Professor Shi-Gang Sun in 2003. Following his graduate studies, he began working as a postdoctoral associate in the area of synthesis and property studies of metal nanoclusters at the University of California-Santa Cruz. He is currently a full professor at the School of Chemistry and Pharmaceutical Sciences, Guangxi Normal University. His research interests include electroanalytical chemistry, surface electrochemistry, electrocatalysis, photoelectrocatalysis, and the controlled synthesis, characterization, and application of nanomaterials in energy storage and conversion.




