Lightweight diamond/Cu interface tuning for outstanding heat conduction
Abstract
With rapid developments in the field of very large-scale integrated circuits, heat dissipation has emerged as a significant factor that restricts the high-density integration of chips. Due to their high thermal conductivity and low thermal expansion coefficient, diamond/Cu composites have attracted considerable attention as a promising thermal management material. In this study, a surface tungsten carbide gradient layer coating of diamond particles has been realized using comprehensive magnetron sputtering technology and a heat treatment process. Diamond/Cu composites were prepared using high-temperature and high-pressure technology. The results show that, by adjusting the heat treatment process, tungsten carbide and di-tungsten carbide are generated by an in situ reaction at the tungsten–diamond interface, and W–WC–W2C gradient layer-coated diamond particles were obtained. The diamond/Cu composites were sintered by high-temperature and high-pressure technology, and the density of surface-modified diamond/Cu composites was less than 4 g cm−3. The W–WC–W2C@diamond/Cu composites have a thermal diffusivity as high as 331 mm2 s−1, and their thermal expansion coefficient is as low as 1.76 × 10−6 K−1. The interface coherent structure of the gradient layer-coated diamond/copper composite can effectively improve the interface heat transport efficiency.
1 INTRODUCTION
With the rapid development of electronic packaging technology, electronic components have become more miniaturized and lightweight. Efficient heat dissipation has become an emergent issue.1-6 At present, commonly used heat dissipation packaging materials include metal-based materials, polymer-based composites, and ceramic-based materials.7 Composites of diamond and high-thermal-conductivity metal with high thermal conductivity and an adjustable thermal expansion coefficient have been identified as very promising electronic packaging materials. Compared with diamond/aluminum and diamond/silver, diamond/copper composites have higher thermal conductivity and a lower thermal expansion coefficient, so they have more large-scale application prospects.8 However, diamond and copper (Cu) have higher interfacial energies, resulting in neither wetting nor a chemical reaction between diamond and Cu.9 These factors affect the bonding between diamond and Cu drastically, such that the actual thermal conductivity is much lower than the theoretical value.10 The contact angle between diamond and liquid Cu can reach up to 131.5° at high temperatures, such as 1200°C.11 To improve the bonding of the diamond–Cu interface, an interfacial bonding layer can be introduced at the interface through metallization of the diamond surface10 and metal matrix alloying.12 Commonly used transition layer metals include Chromium (Cr),12-14 Titanium (Ti),5 Zirconium (Zr),8, 15 Boron (B),16-18 and Tungsten (W).10, 19, 20 For instance, adding 1.5 wt% Ti to Cu metal was reported to improve the interface bonding between the copper matrix and diamond, in which the thermal conductivity of the Cu–diamond (55 vol%) composite prepared by hot forging can reach up to 410 W m−1 K−1.5 In another study, it was reported that Cu-3 wt% Cr/diamond (55 vol%) composite materials have a thermal conductivity of 433 W m−1 K−1.13 When using metal matrix alloying to introduce the interface layer, a part of the coating metal is often retained in the matrix, reducing the thermal conductivity of the metal matrix. Therefore, researchers prefer to use diamond surface metallization to introduce the interface layer.21
Techniques for diamond surface metallization include thermal diffusion, sol–gel,22 salt bath plating, magnetron sputtering,15 and chemical vapor deposition.23 It is difficult to control the uniformity and phase composition of the coating with sol–gel, salt bath coating, and thermal diffusion methods. However, using the magnetron sputtering method, coated diamond particles can be produced on a large scale. The coating is flat and uniform, and the thickness of the coating can be controlled, so it has wide application prospects. When W and Ti coatings with the same thickness (100 nm) are coated on the surface of diamond particles by magnetron sputtering, the thermal conductivity of the Cu/W@diamond (55 vol%) composite prepared by pressure infiltration can reach 530 W m−1 K−1, which is higher than that of the Cu/Ti@diamond (55 vol%) composite.24 In particular, W coating layers have lower solubility in the Cu matrix compared to other coating metals, and W metal has relatively high intrinsic thermal conductivity and high phonon velocity. The thermal conductivity of W-coated diamond particle-reinforced copper matrix composites can theoretically reach more than 900 W m−1 K−1.10
At present, the main methods for preparing diamond/Cu composites include vacuum hot pressing,20 discharge plasma sintering,19 gas infiltration, high-temperature and high-pressure sintering,8, 17 and so forth. It is widely known that magnetron sputtering technology and high-temperature and high-pressure technology have been applied in many industrial fields, and the use of these technologies will enable surface modification of diamond particles and further preparation of composite heat dissipation materials that are more conducive to industrial application.
Based on the above analysis, gradient W–WC–W2C layer-coated diamond particles were prepared by combining magnetron sputtering and heat treatment. A light-weight and high-thermal-conductivity diamond–Cu composite was prepared using high-temperature and high-pressure technology. A coherent interface exists between Cu and the gradient layer-coated diamond, which effectively improves the electron–phonon coupling thermal transport efficiency between the interfaces.
2 EXPERIMENTAL
2.1 Surface modification of diamond particles
In this experiment, diamond was provided by Henan Huanghe Whirlwind Co., China, with an average particle size of about 140 μm. The diamond particles used in this experiment included two kinds of regular and irregular diamond particles. The irregular diamond particles were crushed diamond materials. Cu powder with a purity of 99.99 wt% was used as the metal matrix material.
First, the diamond particles were pretreated and placed in aqua regia (HCl:HNO3 = 3:1 volume ratio) for 3 h to remove metal contaminants. Then, acetone, alcohol, and deionized water were used to remove organic pollutants of diamond particles for 10 min, after which the treated diamond was dried. The treated diamond was placed on a magnetron sputtering sample table with ultrasonic, vibration, and tumbling functions, and W was deposited on the diamond surface by DC magnetron sputtering. The sputtering current was 1 A, the sputtering vacuum was 3 × 10−3 Pa, the sputtering rate was 0.02–0.08 nm min−1, and the sputtering thickness was about 80 nm. The W-plated diamond was then heat-treated under an argon atmosphere at 700–1050°C for 1 h, and the heating rate was 5°C/min.
2.2 Preparation of diamond/Cu composites
The modified diamond particles and Cu powder were uniformly mixed at different mass ratios, and the mixed samples were pressed into a Φ10 mm × 2 mm cylinder using metal cold grinding tools. Finally, the sample was exothermically assembled into a high-pressure chamber. The high-pressure equipment used in the high-temperature and high-pressure process included a hinged hexahedral jacking press (UDS650). The temperature required for the experiment was achieved by heating the graphite tube. The preparation temperatures were 950°C, 1050°C, 1150°C, 1250°C, and 1350°C, the pressure was 6 GPa, and the sintering time was 10 min. The prepared samples were polished, cleaned, and dried. The preparation process is shown in Figure 1.
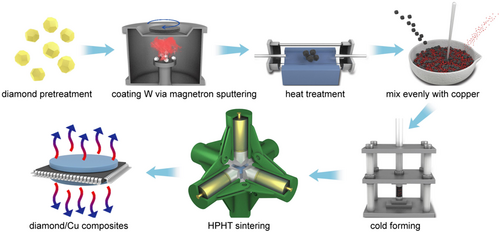
2.3 Sample characterization
3 RESULTS AND DISCUSSION
3.1 Microstructure and phase analyses of diamond before and after modification
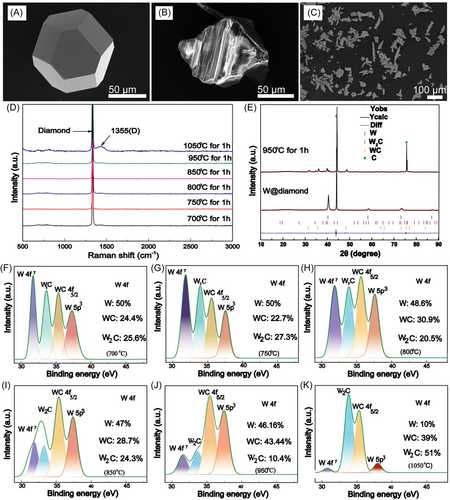
The Gibbs free energies of Formulas (2), (3), and (4) are −43.9, −11.6, and −47.4 kJ mol−1, respectively. According to the calculated Gibbs free energy, after heat treatment, the carbon atoms of diamond preferentially form W2C. The carbon atoms of heated diamond continue to diffuse into the coating and react with W2C to form WC, so the XRD spectrum reveals that some portion of W in the coating is transformed into WC and W2C after heat treatment.
The chemical composition and bonding of W-plated diamond after heat treatment were also studied by X-ray photoelectron spectroscopy (XPS). As shown in Figure 2F–K, the W 4f XPS is fitted by four peaks at 31.3, 34, 35.1, and 37.0 eV, corresponding to W 4f7, W2C, WC, and W 5p3.25 These XPS data further show that the composition of the coating includes W, WC, and W2C. The contents of W, WC, and W2C of the coating changed significantly at different heat treatment temperatures. At the heat treatment temperature of 950°C, the contents of W, WC, and W2C of the coating were 46.16%, 43.44%, and 10.4%, respectively. However, when the heat treatment temperature was increased to 1050°C, the contents of W, WC, and W2C of the coating were 10%, 39%, and 51%, respectively. According to the analysis of XRD and XPS data, the gradient W–WC–W2C layer was coated on the surface of diamond particles by magnetron sputtering combined with heat treatment.
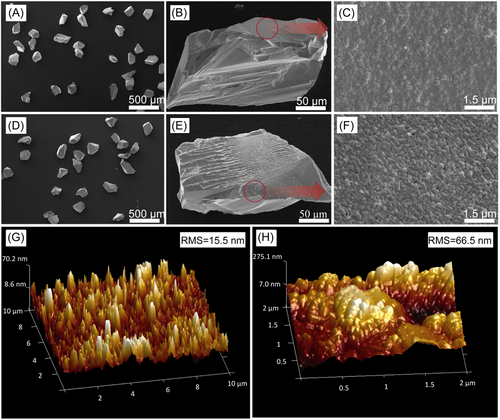
Figure 3A–C shows that metal W is deposited on the diamond surface by magnetron sputtering. The coating is flat and uniform, there is almost no leakage area, and many fine particles are stacked on the bottom layer before heat treatment. The granular layer is composed of discontinuous metal W particles, similar to isolated islands. Figure 3D–F shows that almost no coating falls off after heat treatment, and the connections between large particles are uniform and continuous. Figure 3G,H shows that the root mean square roughness of the diamond surface changes from 15.5 to 66.5 nm after heat treatment. After heat treatment, the coating shows better continuity and higher roughness, and the gradient W–WC–W2C layer coating more easily forms tight connections with Cu.
3.2 Characterization of the microstructure and morphology of diamond/Cu composites
Figure 4A shows the density and relative density of diamond/Cu composites with a diamond content of 90% prepared by high-temperature and high-pressure sintering at different temperatures and at 6 GPa. The density of surface-modified diamond/Cu composites was less than 4 g cm−3. On comparing the relative density of the samples before and after diamond surface modification, we found that surface modification of diamond particles is beneficial to improving the relative density of diamond/Cu composites. When the sintering temperature was 1350°C, the relative density of surface-modified diamond/Cu composites could reach 99.08%. Figure 4B shows the XRD pattern of the composite prepared with modified and unmodified diamond. It can be seen that the main components of the W–WC–W2C gradient layer-coated diamond/Cu composite are diamond, W, WC, and W2C. Figure 4C–F shows the SEM image of the fracture surface of the diamond/Cu composite. As shown in Figure 4C,D, a large number of diamonds fell off portions of the unmodified diamond–Cu composite, and there was a large gap at the diamond–Cu bonding surface. It can be seen from Figure 4E,F that there is no diamond falling off metal copper matrix in the W–WC–W2C gradient layer-coated diamond/Cu composite, and the diamond particle is closely bonded to the Cu matrix. This is due to the gap at the unmodified diamond–Cu interface, which has a large wetting angle. The introduction of the coating after modification improved the bonding of the interface between diamond and Cu.26-29 The W–WC–W2C gradient layer coating has a smaller wetting angle and a relatively high roughness, which increases the holding force of the Cu matrix on the diamond. Also, the interface bonding of the W–WC–W2C gradient layer-coated diamond/Cu composites with different diamond contents is compact (as shown in Figure S1).
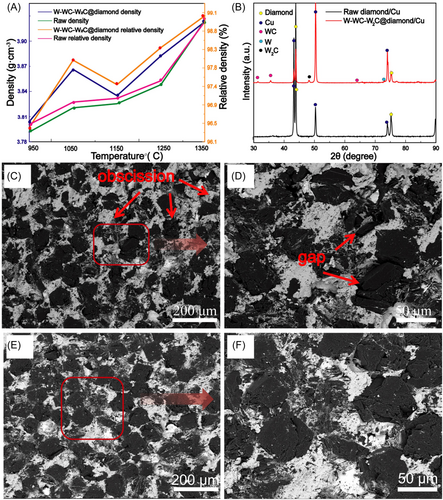
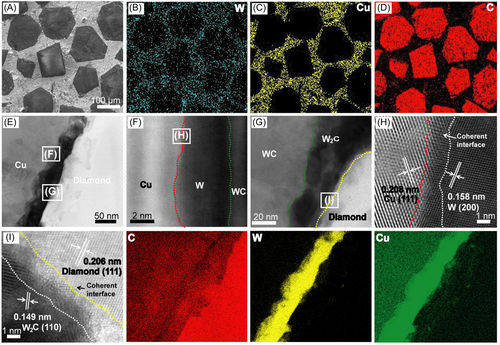
Figure 5A shows that diamond is uniformly distributed and closely bonded to the Cu matrix. Figure 5B,C shows a scanning map of the distribution of Cu and W elements in the interface bonding area between the diamond and the Cu matrix. From the distribution of W elements shown in Figure 5, it is clear that most W elements are concentrated at the diamond–Cu interface, and a small amount of W diffuses into the Cu matrix, which is due to the lower solubility of W, WC, and W2C in the Cu matrix. The reduced diffusion of the coating in the Cu matrix and the reduced diffusion of the coating metal have less effect on the intrinsic thermal conductivity of the Cu matrix. Similarly, the interface of the crushed diamond/Cu composite material is also tightly bonded (Figure S2). Figure 5D shows the TEM bright-field image and the elemental distribution of the interface layer of the W–WC–W2C gradient layer-coated diamond/Cu composites. The interface layer is composed of three different regions of Cu, interface coating layer, and diamond. The W element is mainly concentrated at the interface between diamond and Cu, and there is almost no diffused Cu throughout the matrix. The coating is evenly distributed between diamond and Cu (Figure S3). The interface between diamond and Cu is tightly bonded by the gradient W–WC–W2C coating layer, and there is no gap between the interface, as can be seen from the STEM annular bright-field (ABF) images in Figure 5E,F. Also, we observed a coherent interface between Cu/W (Figure 5E) and W2C/diamond (Figure 5F). The result further confirms the conclusion that the composition of the coating in Figure 2 is W–WC–W2C.
3.3 Thermophysical properties of diamond/Cu composites
Figure 6 shows the thermophysical properties of diamond/Cu composite materials. As shown in Figure 6A, the diamond/Cu composite has the best heat transfer performance at the heat treatment temperature of tungsten-plated diamond of 950°C under certain preparation conditions (preparation temperature 1050°C, diamond content 90%). With increasing heat treatment temperature, the thermal diffusivity of the sample increases first and then decreases. At the heat treatment temperature of surface-modified diamond particles of 950°C, the thermal diffusion coefficient of the sample shows the highest value. Figure 6B shows the thermal diffusivity of diamond/Cu composites before and after diamond modification at different preparation temperatures. The results show that the thermal diffusivity values of W–WC–W2C gradient layer-modified diamond/Cu composites are much higher than those of unmodified diamond/Cu composites. After gradient W–WC–W2C layer modification of diamond, the highest thermal diffusivity is 331 mm2 s−1. Figure 6C shows the thermal conductivity of diamond/Cu composites before and after diamond modification at different preparation temperatures. The variation of the thermal conductivity of diamond/Cu composites is consistent with the thermal diffusion coefficient. The thermal conductivity values of W–WC–W2C gradient layer-modified diamond/Cu composites are much higher than those of unmodified diamond/Cu composites. Also, the crushed diamond/Cu composite materials could reach a level of thermal conductivity similar to that of the complete crystal form diamond/Cu composites after modification. The highest thermal conductivity of the W–WC–W2C gradient layer-modified diamond/Cu composite is 655 W m−1 k−1.

Electronic packaging requires heat dissipation materials to have high thermal conductivity and lightweight properties. To better evaluate the comprehensive performance of heat dissipation materials, we therefore propose a new heat dissipation material parameter-thermal conductivity density (TCD) ratio, calculated as TCD = Tc/D, where Tc is the thermal conductivity of the heat dissipation material and D is the density of the heat dissipation material. It can be seen from Figure 6D that the TCD value of the gradient W–WC–W2C layer-modified diamond/copper composite is much higher than that of unmodified diamond. This is mainly due to the introduction of a gradient W–WC–W2C layer coating between diamond and Cu to improve the interface bonding and increase thermal conductivity. When the temperature at which the diamond/copper composite is prepared was 1050°C, the TCD value reached 172.8 W m−1 K−1 g−1 cm3. Composites with high diamond contents were prepared under conditions of high temperature and high pressure to reduce sample density. It can be seen from Figure 6E that the thermal expansion coefficient decreases with increasing diamond content. The thermal expansion coefficient can be adjusted by adjusting the diamond content, and the lowest thermal expansion coefficient was 1.76 × 10−6 K−1. The low thermal expansion coefficients of the composites further confirm a strong diamond–Cu connection. It can be seen from Figure 6F that compared with the diamond/Cu composite reported in the literature,6, 8, 10, 13, 15, 16, 18-20, 32, 33 the experimental thermal diffusivity of 311 mm2 s−1 is the highest, and the density of 3.78 g cm−3 is the lowest, which are more in line with the requirements of high thermal diffusion and lightweight electronic packaging materials.
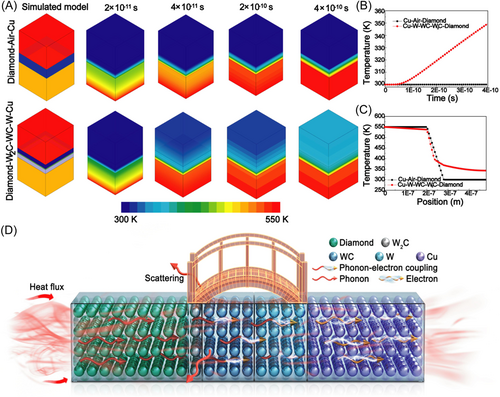
To better understand the interface heat transfer mechanism, the heat transfer between diamond/copper composites was calculated by finite element simulation. The finite element model includes diamond, an intermediate layer, and a copper layer. The preset heat is transferred from diamond to copper through the intermediate layer. The initial temperature used in the model is 300 K. The bottom boundary temperature in the model is 550 K, and other boundaries are adiabatic. Figure 7A–C shows the finite element analysis of the diamond/copper composite under different interface conditions. The simulated heat is transferred into the diamond/copper composite from the diamond side. As shown in Figure 7A, when the diamond/Cu interface is an air layer, the heat mainly concentrates at the interface. Figure 7B shows the change of the center point temperature of the simulated copper module with time. Compared with the uncoated diamond/Cu composite, the simulated center temperature of the Cu module in the gradient W–WC–W2C layer-coated diamond/Cu composite is higher at the same heat transfer time. Figure 7C shows the central axis temperature distribution from diamond through the interface layer to Cu after 4 × 10−10 s. Compared with the interface air layer between diamond and copper, the temperature change of the gradient W–WC–W2C layer-coated diamond/copper composite is more pronounced, and the heat can be transported away efficiently between the interface. Based on the finite element simulation and experimental data, the heat transport mechanism of the gradient W–WC–W2C layer-coated diamond/copper composites is shown in Figure 7D. It is well known that the heat transfer mechanisms of diamond and Cu are quite different. The main hot carrier of diamond is phonon, while Cu mainly relies on electrons for heat transfer. To solve the discontinuous heat transfer problem between different heat carriers, the coherent interface between diamond and copper provides a bridge for the heat transport between phonons and electrons. The gradient layer interface as a bridge reduces the phonon scattering, improves the interface phonon–electron coupling efficiency, and is conducive to the interface hot carrier transport.
4 CONCLUSIONS
A gradient W–WC–W2C layer was coated on the surface of diamond particles by magnetron sputtering combined with heat treatment, and diamond/Cu composites with high TCD were prepared through a high-temperature and high-pressure sintering process. The modification of the diamond surface enabled close bonding between the diamond and the Cu matrix. A coherent interface exists between Cu and gradient layer-coated diamond, which effectively improves the electron–phonon coupling thermal transport efficiency between the interfaces. The density of the gradient W–WC–W2C layer-coated diamond/copper composites was less than 4 g cm−3. The thermal diffusivity of the prepared composite can reach up to 331 mm2 s−1, and the highest thermal conductivity was 655 W m−1 K−1. After surface gradient layer modification of diamond, the thermal conductivity of the diamond–copper composite was increased by 55%, and the TCD was 172.8 W m−1 K−1 g−1 cm3. Therefore, this work provides an innovative and valuable design concept for improving the thermal transport efficiency of different carbon matrix composites and provides a new method support for solving the heat dissipation problem of ultrahigh density electronic packaging.
ACKNOWLEDGMENTS
This work was financially supported by National Natural Science Foundation of China (Grant No. 52072327); the China National Key R&D Program (2021YFB3701802); Scientific and Technological Projects of Henan Province (No. 232102231050); the Higher Education and Teaching Reformation Project (2014SJGLX064); the Project for Work-station of Zhongyuan scholars of Henan Province (Nos. 214400510002, 224400510023); the Science and Technology Major Project of Henan Province (No. 221100230300); the Postgraduate Education Reform and QualityAcademic Degrees & Graduate Education Reform Project of Henan Province (No. 2021SJGLX060Y); the Postgraduate Education Reform and Quality Improvement Project of Henan Province (No. YJS2022JD34); and the Science and Technology on Plasma Physics Laboratory (Grant No. JCKYS2021212010).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.




